2022
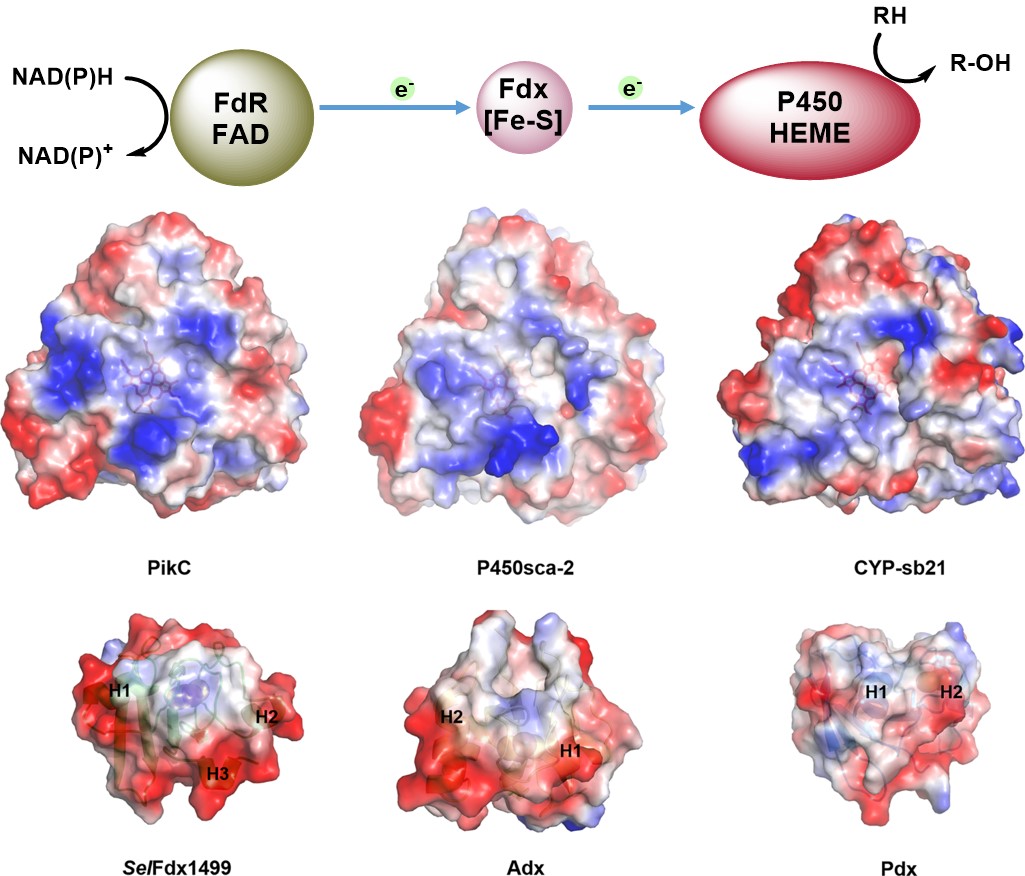
1. Liu, X.#, Li, F.#, Sun, T., Guo, J., Zhang, X., Zheng, X., Du, L., Zhang, W., Ma, L. *, Li, S. Three pairs of surrogate redox partners comparison for Class I cytochrome P450 enzyme activity reconstitution. Commun. Biol 2022, doi.org /10.1038/s42003-022-03764-4. | PDF
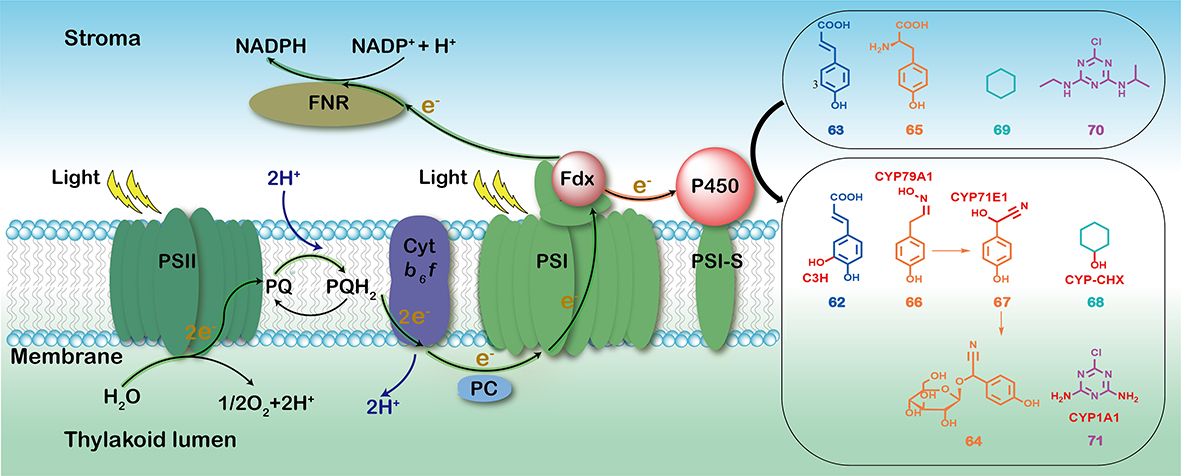
2. Zheng, S. #, Guo, J. #, Cheng, F., Gao, Z., Du, L., Meng, C.*, Li, S.*, Zhang, X.* Cytochrome P450s in algae: Bioactive natural product biosynthesis and light-driven bioproduction. Acta Pharm. Sin. B 2022, doi.org/10.1016/j.apsb.2022.01.013. | PDF

3. Durairaj, P., Li, S.* Functional expression and regulation of eukaryotic cytochrome P450 enzymes in surrogate microbial cell factories. Eng. Microbiol. 2022, doi.org/10.1016/j.engmic.2022.100011. | PDF
4. Wang, Y. #, Wang, P. #, Cao, H., Ding, H., Su, H., Liu, S., Liu, G., Zhang, X., Li, C., Peng, M., Li, F., Li, S., Chen, Y., Chen, X. *, Zhang, Y. * Structure of Vibrio collagenase VhaC provides insight into the mechanism of bacterial collagenolysis. Nat. Commun. 2022, 13(1): 566. | PDF
2021
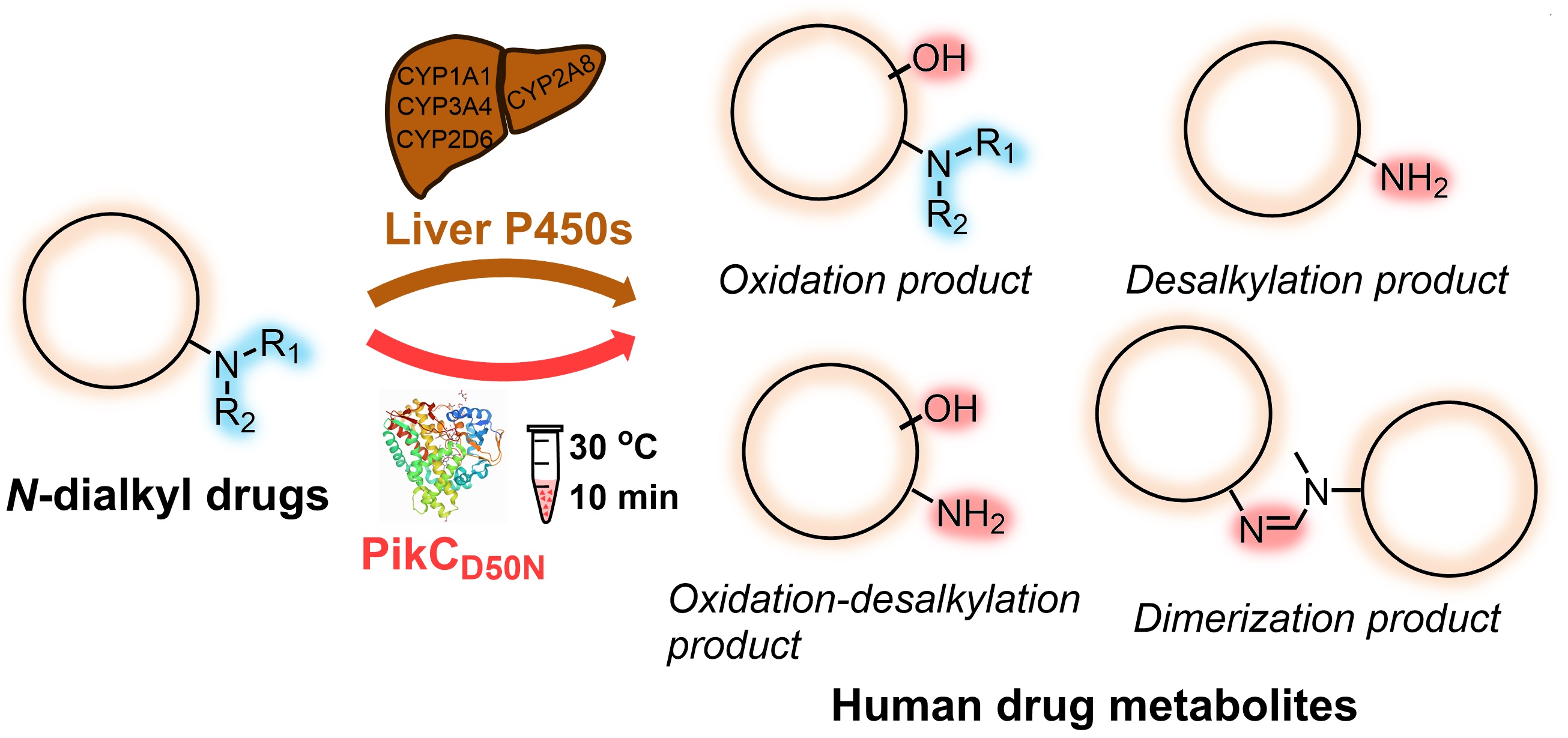
1. Guo, J., Li, F., Cheng, F., Ma, L., Liu, X., Durairaj, P., Zhang, G., Tang, D., Long, X., Zhang, W., Du, L., Zhang, X.*, Li, S. Bacterial biosynthetic P450 enzyme PikCD50N: A potential biocatalyst for the preparation of human drug metabolites. J. Org. Chem. 2021, 86, 14563-14571. | PDF
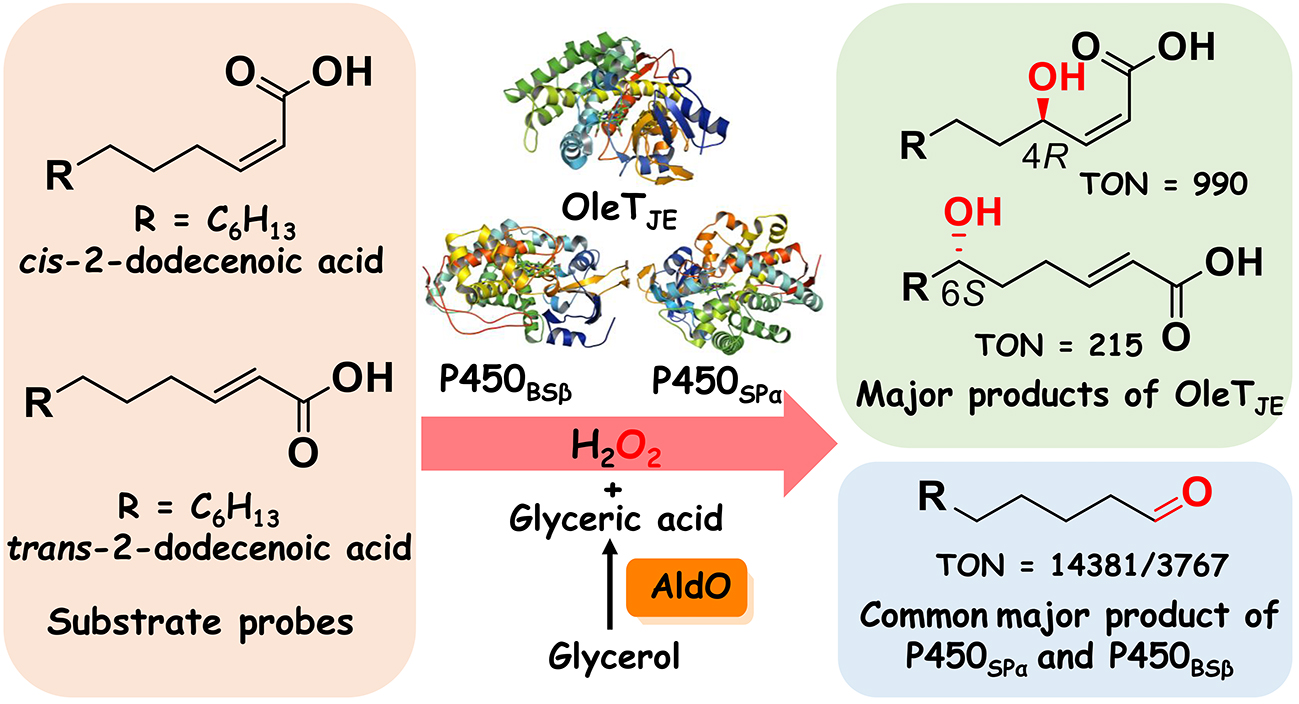
2. Jiang, Y. #, Peng, W. #, Li, Z. #, You, C., Zhao, Y., Tang, D., Wang, B. *, Li, S. * Unexpected reactions of α,β-unsaturated fatty acids provide insight into the mechanisms of CYP152 peroxygenases. Angew. Chem. Intl. Ed. 2021, 60, 24694-24701. | PDF
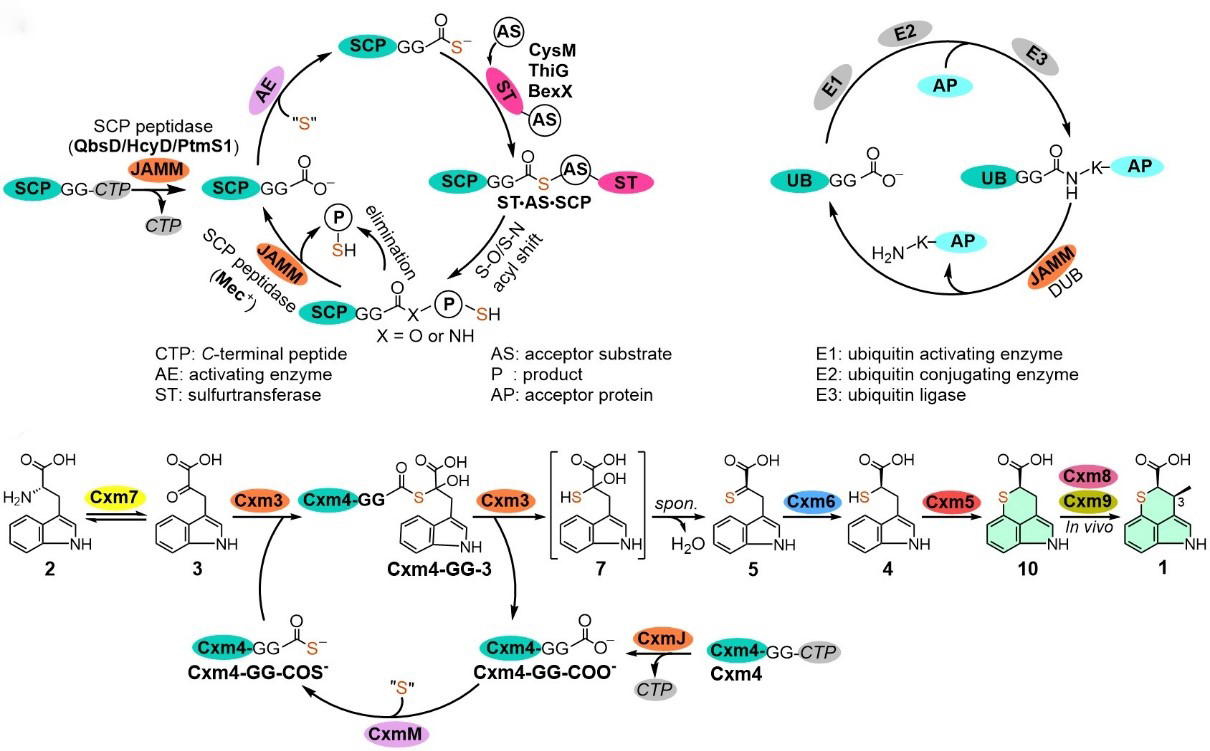



3. Zhang, X., Xu, X., You, C., Yang, C., Guo, J., Sang, M., Geng, C., Cheng, F., Du, L., Shen, Y., Wang, S., Lan, H., Yang, F., Li, Y., Tang, Y., Zhang, Y., Bian, X.*, Li, S.*, Zhang, W.* Biosynthesis of chuangxinmycin featuring a deubiquitinase-like sulfurtransferase. Angew. Chem. Intl. Ed. 2021, 60, 24418-24423. | PDF

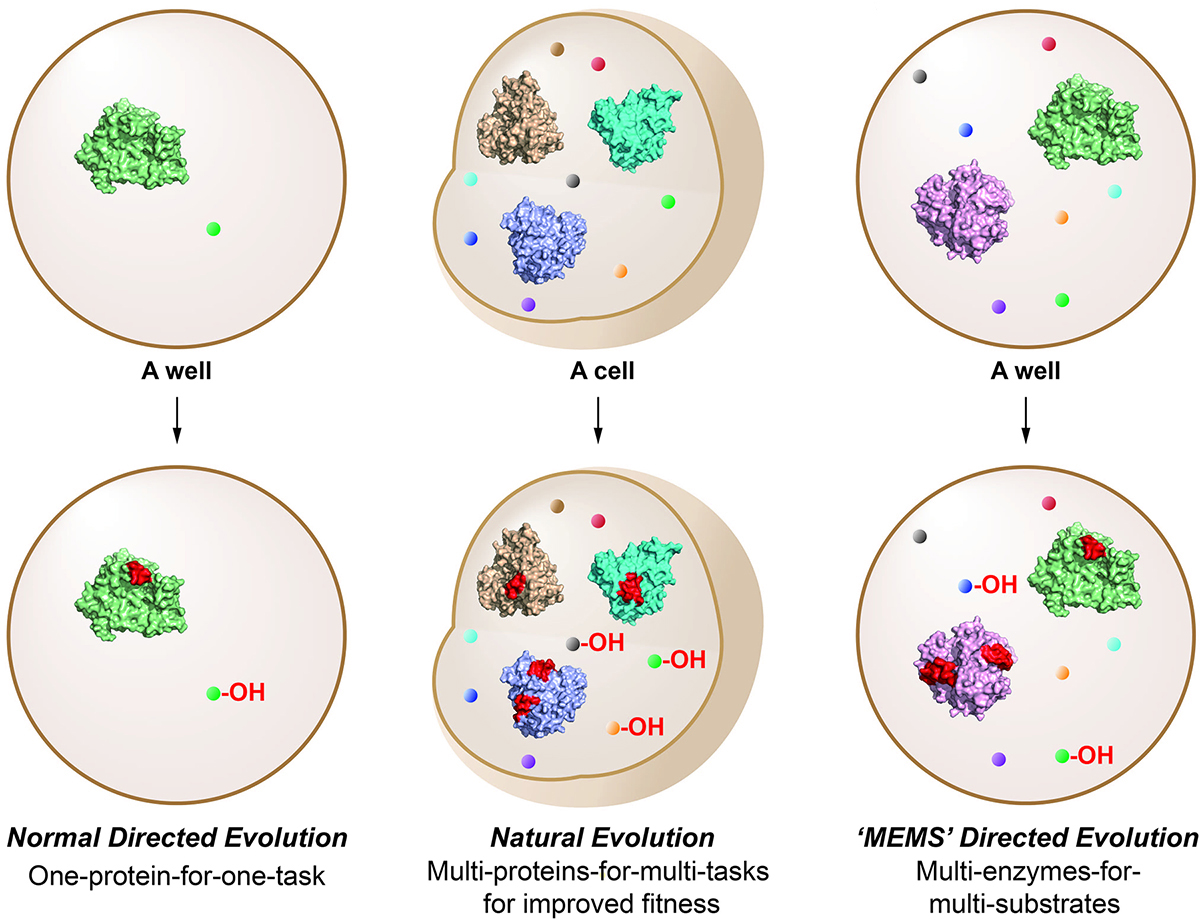
4. Ma, L., Li, F., Zhang, X., Chen, H., Huang, Q., Su, J., Liu, X., Sun, T., Fang, B., Liu, K., Tang, D., Wu, D., Zhang, W., Du, L., Li, S. * Development of MEMS directed evolution strategy for multiplied throughput and convergent evolution of cytochrome P450 enzymes. Sci. China Life Sci. 2021, doi.org/10.1007/s11427-021-1994-1. | PDF

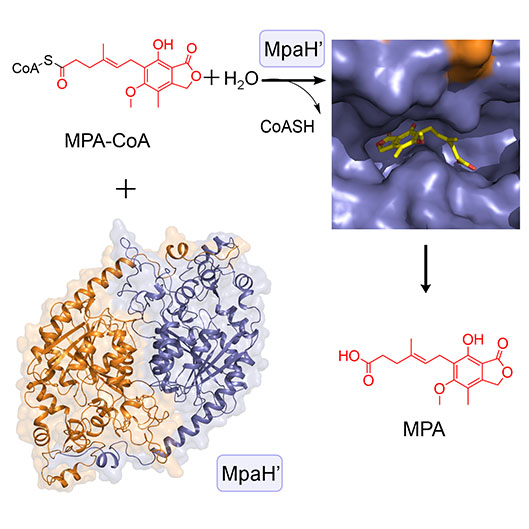
5. You, C., Li, F., Zhang, X., Ma, L., Zhang, Y., Zhang, W., and Li, S. * Structural basis for substrate specificity of the peroxisomal acyl-CoA hydrolase MpaH’involved in mycophenolic acid biosynthesis. FEBS J. 2021, 288, 5768-5780. | PDF

6. Dong, S. #, Chen, J. #, Zhang, X., Guo, F., Ma, L., You, C., Wang, X., Zhang, W., Wan, X., Liu, S., Yao, L., Li, S., Du, L. * and Feng, Y. * Structural basis for selective oxidation of phosphorylated ethylphenols by cytochrome P450 monooxygenase CreJ. Appl. Environ. Microbiol. 2021, 87(11), e00018-21. | PDF

7. Zhang, X., Guo, J., Cheng, F., and Li, S. * Cytochrome P450 enzymes in fungal natural product biosynthesis. Nat. Prod. Rep. 2021, 38, 1072. | PDF
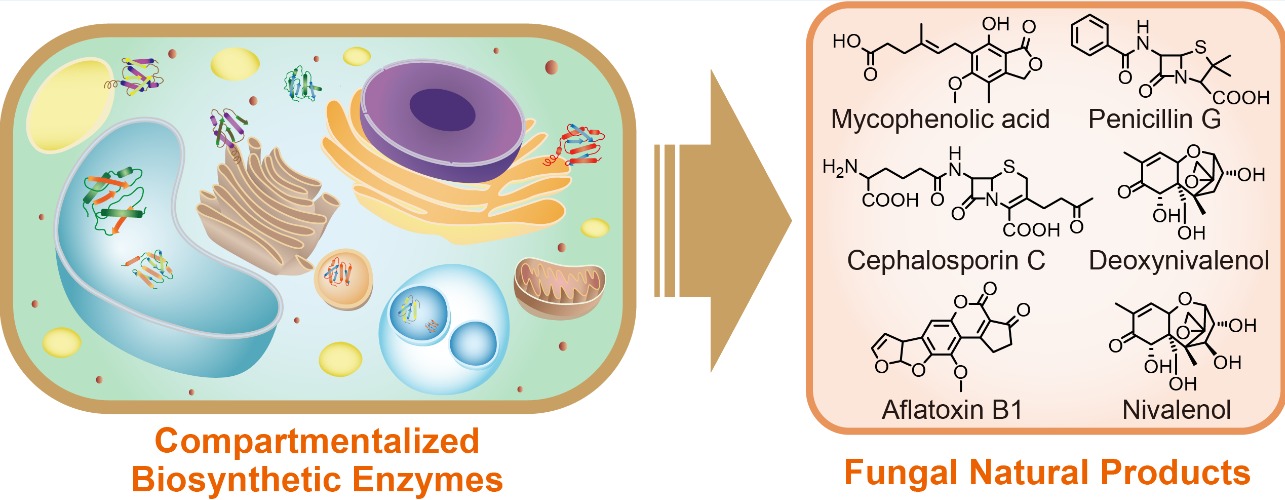
8. Du, L., and Li, S. * Compartmentalized biosynthesis of fungal natural products. Curr. Opin. Biotechnol. 2021, 69, 128-135. | PDF

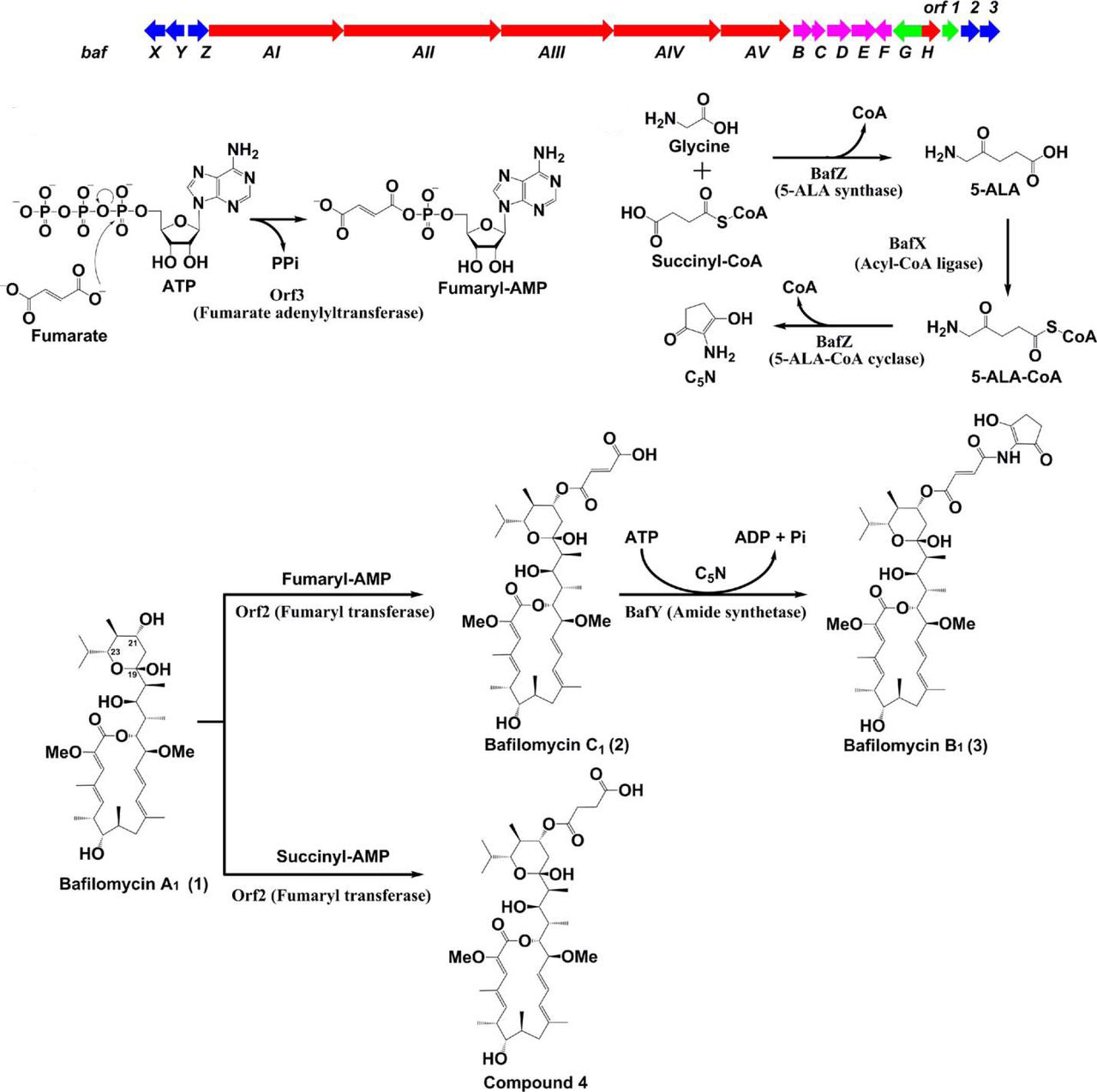
9. Li, Z., Li, S., Du, L., Zhang, X., Jiang, Y., Liu, W., Zhang, W., and Li, S. * Engineering Bafilomycin High-Producers by Manipulating Regulatory and Biosynthetic Genes in the Marine Bacterium Streptomyces lohii. Mar. Drugs 2021, 19, 29. | PDF

10. Zhao, W. #, Duan, Y. #, Li, H., Li, S., Shen, Y., Zhang, Y., Li, Y., Tang, Y. * Triazole/thiadiazole substituted 4'-demethylepipodophyllotoxin derivatives induced apoptosis in HeLa cells by up-regulating TMEM133. Eur. J. Pharmacol. 2021, 905, 174189. | PDF
11. Li, C. #, Wang, X. #, Chen, X., Sheng, Q., Zhang, S., Wang, P., Quareshy M., Rihtman, B., Shao, X., Gao, C., Li, F., Li, S., Zhang, W., Zhang, X., Yang, G., Todd, J. D., Chen, Y., Zhang, Y. * A novel ATP dependent dimethylsulfoniopropionate lyase in bacteria that releases dimethyl sulfide and acryloyl-CoA. elife 2021, 10, e64045. | PDF
12. Cheng, Y., Zhu, S., Guo, C., Xie, F., Jung, D., Li, S., Zhang, W., * He, S. * Microbulbifer hainanensis sp. nov., a moderately halopilic bacterium isolated from mangrove sediment. Antonie van Leeuwenhoek 2021, 114(7), 1033-1042. | PDF
13. Xie, B. #, Rong, J. #, Tang, B. #, Wang, S. #, Liu, G., Qin, Q., Zhang, X., Zhang, W., She, Q., Chen, Y., Li, F., Li, S., Chen, X., Luo, H. *, Zhang, Y. * Evolutionary Trajectory of the Replication Mode of BacterialReplicons. mBio 2021, 12(1), e02745-20. | PDF
11. Li, C. #, Wang, X. #, Chen, X., Sheng, Q., Zhang, S., Wang, P., Quareshy M., Rihtman, B., Shao, X., Gao, C., Li, F., Li, S., Zhang, W., Zhang, X., Yang, G., Todd, J. D., Chen, Y., Zhang, Y. * A novel ATP dependent dimethylsulfoniopropionate lyase in bacteria that releases dimethyl sulfide and acryloyl-CoA. elife 2021, 10, e64045. | PDF
12. Cheng, Y., Zhu, S., Guo, C., Xie, F., Jung, D., Li, S., Zhang, W., * He, S. * Microbulbifer hainanensis sp. nov., a moderately halopilic bacterium isolated from mangrove sediment. Antonie van Leeuwenhoek 2021, 114(7), 1033-1042. | PDF
13. Xie, B. #, Rong, J. #, Tang, B. #, Wang, S. #, Liu, G., Qin, Q., Zhang, X., Zhang, W., She, Q., Chen, Y., Li, F., Li, S., Chen, X., Luo, H. *, Zhang, Y. * Evolutionary Trajectory of the Replication Mode of BacterialReplicons. mBio 2021, 12(1), e02745-20. | PDF
2020
1. Ye, Y. #, Du, L. #, Zhang, X., Newmister, S. A., McCauley, M., Alegre-Requena, J. V., Zhang, W., Mu, S., Minami, A., Fraley, A. E., Adrover-Castellano, M. L., Carney, N. A., Shende, V. V., Qi, F., Oikawa, H., Kato, H., Tsukamoto, S., Paton, R. S., Williams, R. M.*, Sherman, D. H.*, and Li, S.* Fungal-derived Brevianamide assembly by a stereoselective semi-pinacolase. Nat. Catal. 2020, 3(6), 497-506. | PDF

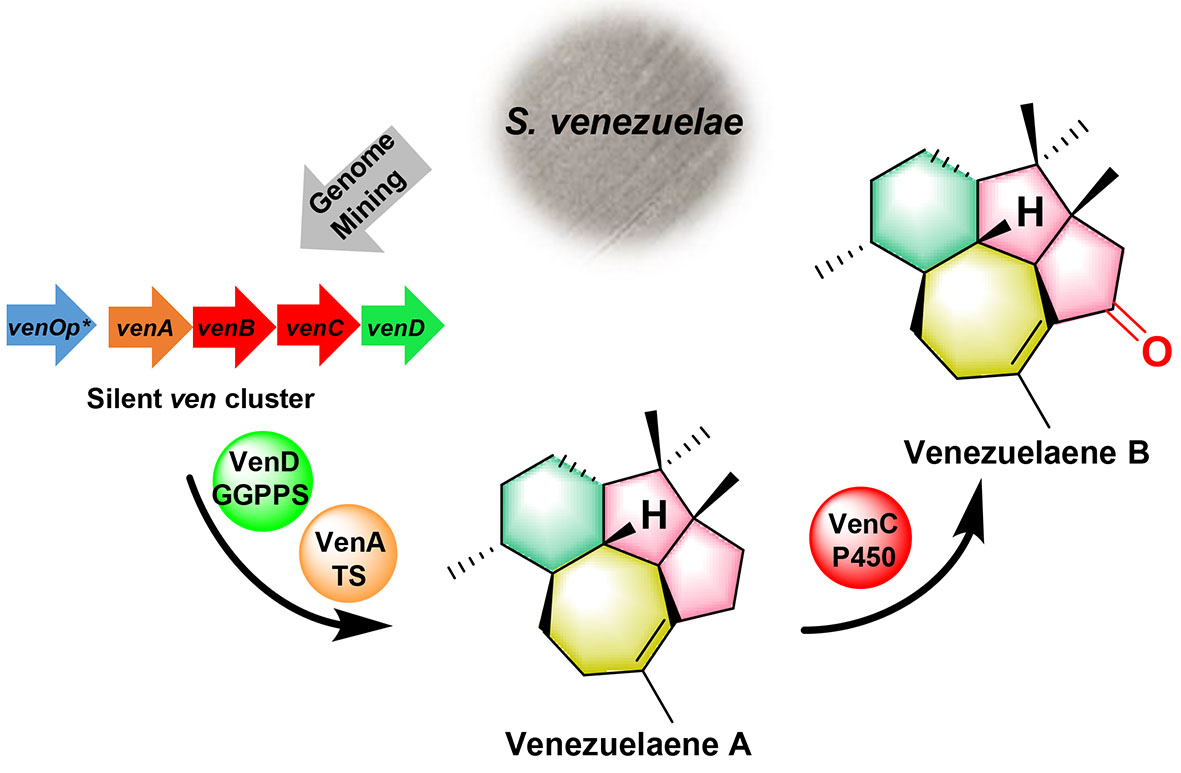
2. Li, Z., Jiang, Y., Zhang, X., Chang, Y., Li, S., Zhang, X., Zheng, S., Geng, C., Men, P., Ma, L., Yang, Y., Gao, Z., Tang, Y., and Li, S.* Fragrant venezuelaenes A and B with A5−5−6−7 tetracyclic skeleton: discovery, biosynthesis, and mechanisms of central catalysts. ACS Catal. 2020, 10, 5846-5851. | PDF
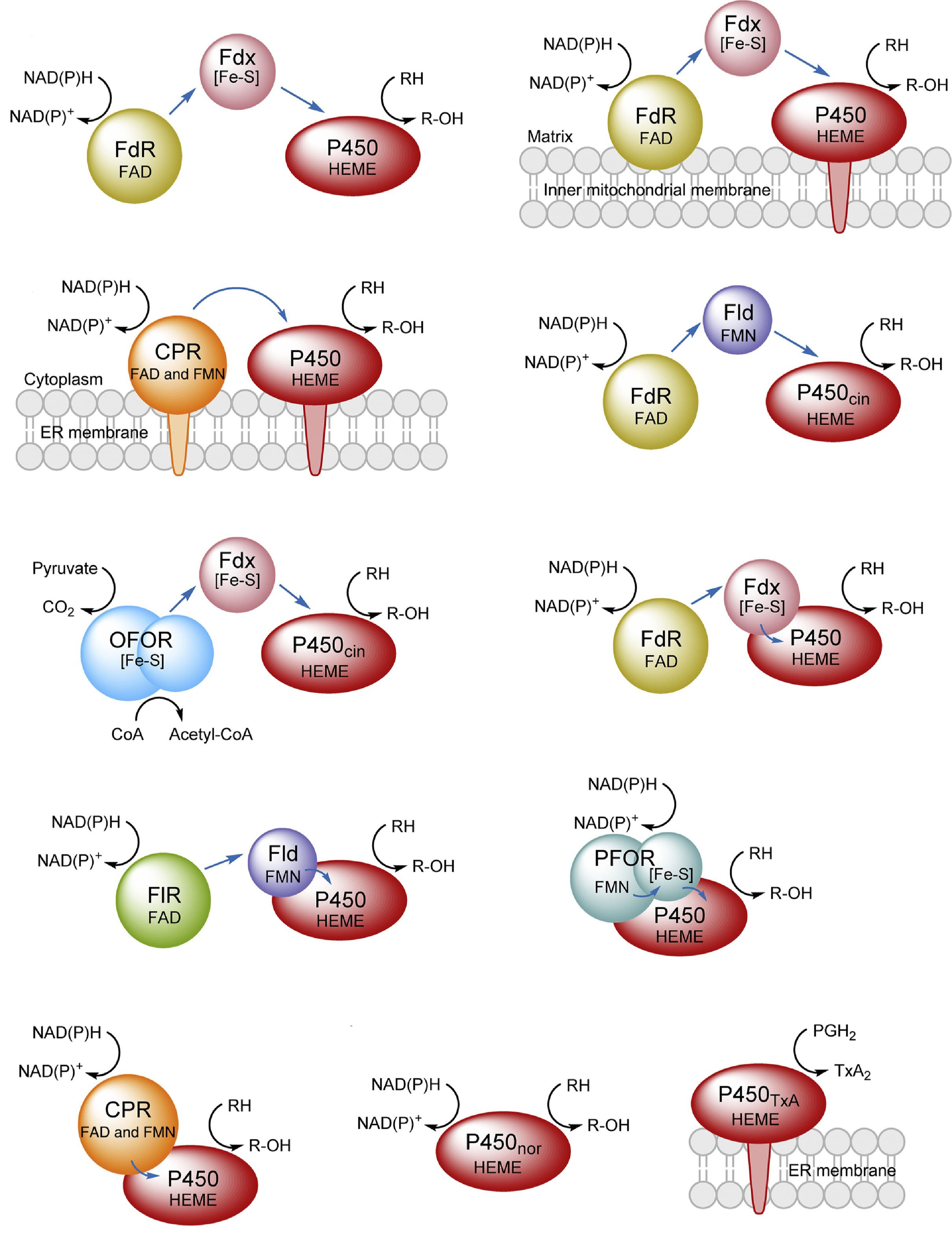
3. Li, S.*, Du, L., and Bernhardt, R.* Redox partners: Function modulators of bacterial P450 enzymes. Trends Microbiol. 2020, 28(6): 445-454. | PDF
4. Jiang, Y., Li, Z., Zheng, S., Xu, H., Zhou, Y. J., Gao, Z., Meng, C. *, and Li, S.* Establishing an enzyme cascade for one-pot production of α-olefns from low-cost triglycerides and oils without exogenous H2O2 addition. Biotechnol. Biofuels 2020, 13, 52. | PDF

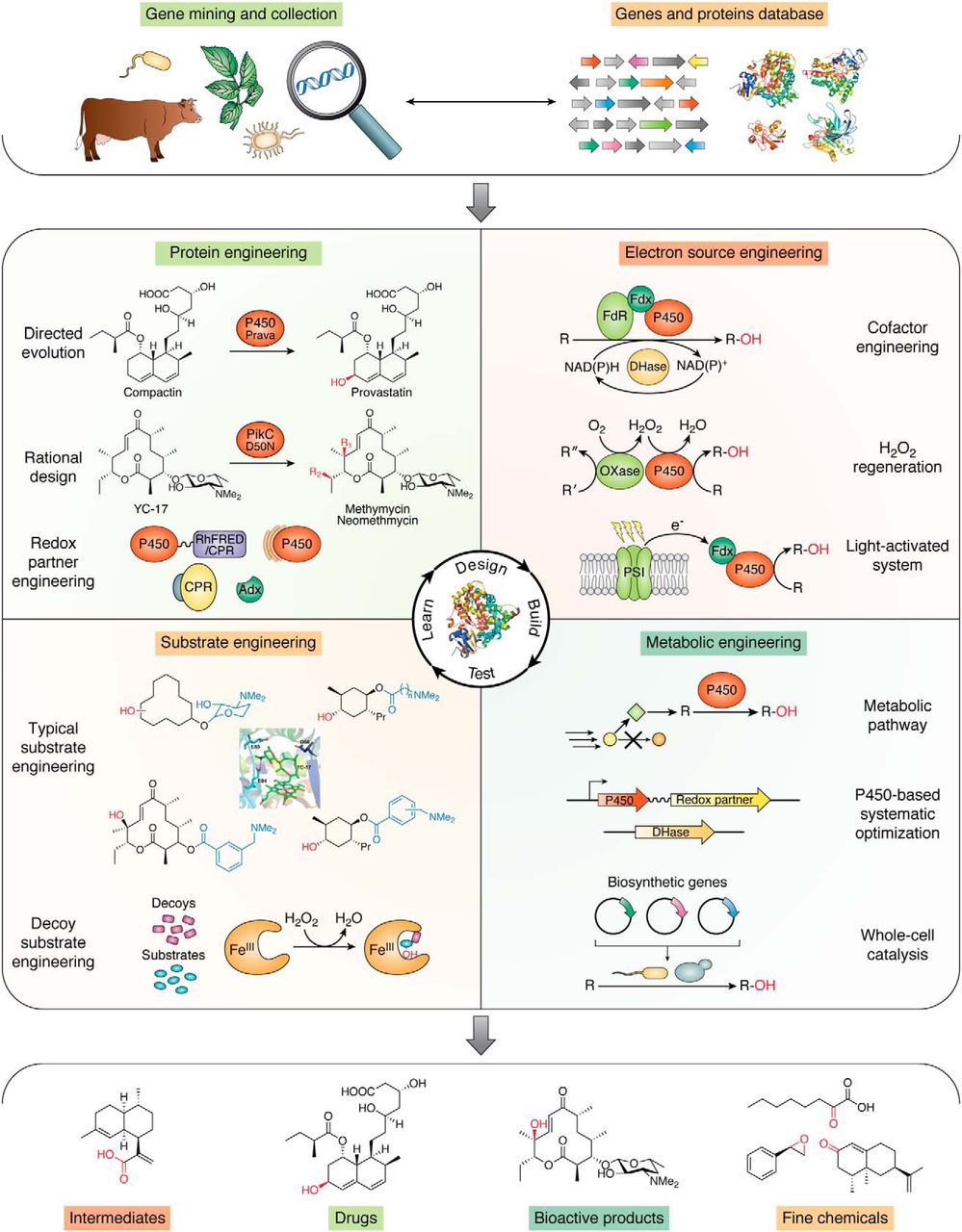
5. Li, Z. #, Jiang, Y. #, Guengerich, F. P., Ma, L., Li, S., and Zhang, W. * Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications. J. Biol. Chem. 2020, 295(3), 833-849. | PDF
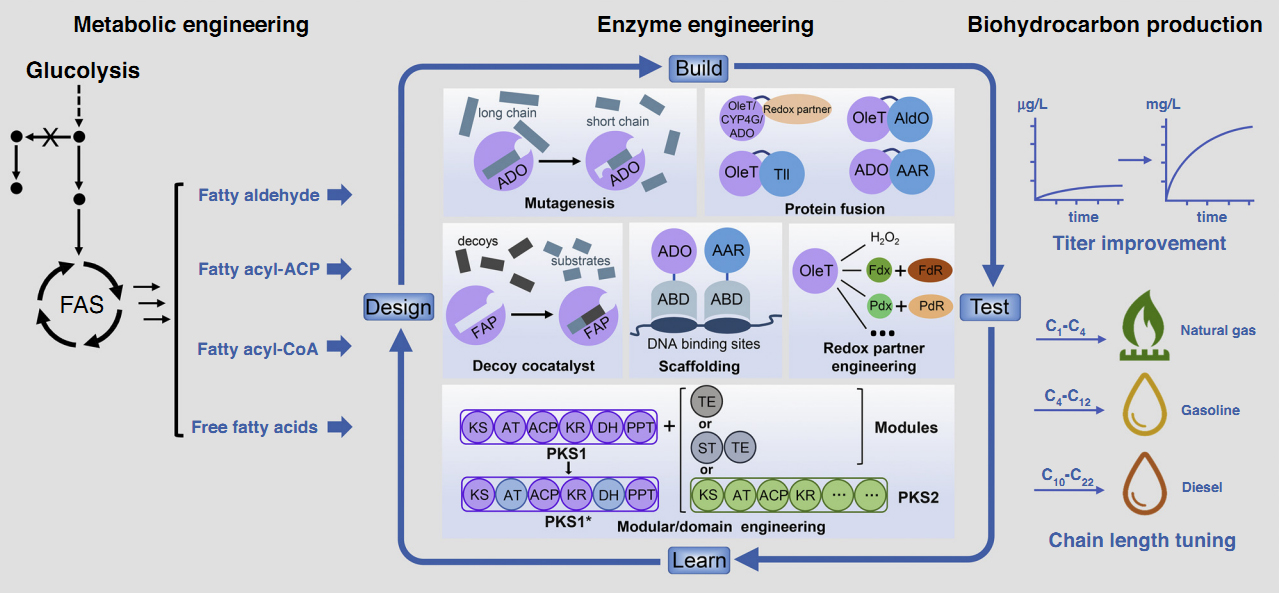
9. Yang, S., Cao, X., Yu, W., Li, S., Zhou, Y. J. * Efficient targeted mutation of genomic essential genes in yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2020, 104(7):3037-3047. | PDF
10. Ding, L., Xu, P., Zhang, W., Yuan, Y., He, X., Su, D., Shi, Y., Naman, C. B., Yan, X., Wu, B., Lazaro, J. E. H., Li, S., and He, S.* Three new diketopiperazines from the previously uncultivable marine bacterium Gallaecimonas mangrovi HK-28 cultivated by iChip. Chem. Biodivers. 2020, doi.org/10.1002/cbdv.202000221. | PDF
11. Fraley, A. E., Tran, H. T., Kelly, S. P., Newmister, S. A., Tripathi, A., Kato, H., Tsukamoto, S., Du, L., Li, S., Williams, R. M.*, and Sherman, D. H.* Flavin-dependent monooxygenases NotI and NotI’ mediate spiro-oxindole formation in biosynthesis of the notoamides. Chembiochem 2020, doi.org/10.1002/cbic.202000004. | PDF
12. Jia, K.#, Zhan, X.#, Li, H., Li, S., Shen, Y., Qi, Q., Zhang, Y., Li, Y., and Tang, Y.* A novel podophyllotoxin derivative with higher anti-tumor activity produced via 4︐-demethylepipodophyllotoxin biotransformation by Penicillium purpurogenum. Process Biochem. 2020, doi.org/10.1016/j.procbio.2020.05.006. | PDF
13. Gao, X., Zhang, X., Chen, W., Li, J., Yang, W., Zhang, X., Li, S., Liu, C.* Transcriptome analysis of Paris polyphylla var. yunnanensis illuminates the biosynthesis and accumulation of steroidal saponins in rhizomes and leaves. Phytochemistry 2020, 178, 112460.| PDF
14. Zhao, W. #, Cong, Y. #, Li, H., Li, S., Shen, Y., Qi, Q., Zhang, Y., Li, Y., Tang, Y. * Challenges and potential for improving the druggability of podophyllotoxin-derived drugs in cancer chemotherapy. Nat. Prod. Rep. 2020, doi. 10.1039/d0np00041h.| PDF

3. Li, S.*, Du, L., and Bernhardt, R.* Redox partners: Function modulators of bacterial P450 enzymes. Trends Microbiol. 2020, 28(6): 445-454. | PDF


5. Li, Z. #, Jiang, Y. #, Guengerich, F. P., Ma, L., Li, S., and Zhang, W. * Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications. J. Biol. Chem. 2020, 295(3), 833-849. | PDF

6. Liu, K., and Li, S.* Biosynthesis of fatty acid derived hydrocarbons: perspectives on enzymology and enzyme engineering. Curr. Opin. Biotech. 2020, 62, 7-14. | PDF

7. Yuan, Y., He, X., Wang, T., Zhang, X., Li, Z., Xu, X., Zhang, W., Yan, X.*, Li, S.*, He S.*Efficient preparation of bafilomycin A1 from marine Streptomyces lohii fermentation using three-phase extraction and high-speed counter-current chromatography. Mar. Drugs 2020, 18, 322. | PDF
8. Li, F.*, Ma, L., Zhang, X., Chen, J., Qi, F., Huang, Y., Qu, Z., Yao, L., Zhang, W., Kim, E.-S.*, and Li, S.* Structure-guided manipulation of the regioselectivity of the cyclosporine A hydroxylase CYP-sb21 from Sebekia benihana. Synth. Syst. Biotechnol. 2020, 5, 236-243. | PDF
8. Li, F.*, Ma, L., Zhang, X., Chen, J., Qi, F., Huang, Y., Qu, Z., Yao, L., Zhang, W., Kim, E.-S.*, and Li, S.* Structure-guided manipulation of the regioselectivity of the cyclosporine A hydroxylase CYP-sb21 from Sebekia benihana. Synth. Syst. Biotechnol. 2020, 5, 236-243. | PDF

9. Yang, S., Cao, X., Yu, W., Li, S., Zhou, Y. J. * Efficient targeted mutation of genomic essential genes in yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2020, 104(7):3037-3047. | PDF
10. Ding, L., Xu, P., Zhang, W., Yuan, Y., He, X., Su, D., Shi, Y., Naman, C. B., Yan, X., Wu, B., Lazaro, J. E. H., Li, S., and He, S.* Three new diketopiperazines from the previously uncultivable marine bacterium Gallaecimonas mangrovi HK-28 cultivated by iChip. Chem. Biodivers. 2020, doi.org/10.1002/cbdv.202000221. | PDF
11. Fraley, A. E., Tran, H. T., Kelly, S. P., Newmister, S. A., Tripathi, A., Kato, H., Tsukamoto, S., Du, L., Li, S., Williams, R. M.*, and Sherman, D. H.* Flavin-dependent monooxygenases NotI and NotI’ mediate spiro-oxindole formation in biosynthesis of the notoamides. Chembiochem 2020, doi.org/10.1002/cbic.202000004. | PDF
12. Jia, K.#, Zhan, X.#, Li, H., Li, S., Shen, Y., Qi, Q., Zhang, Y., Li, Y., and Tang, Y.* A novel podophyllotoxin derivative with higher anti-tumor activity produced via 4︐-demethylepipodophyllotoxin biotransformation by Penicillium purpurogenum. Process Biochem. 2020, doi.org/10.1016/j.procbio.2020.05.006. | PDF
13. Gao, X., Zhang, X., Chen, W., Li, J., Yang, W., Zhang, X., Li, S., Liu, C.* Transcriptome analysis of Paris polyphylla var. yunnanensis illuminates the biosynthesis and accumulation of steroidal saponins in rhizomes and leaves. Phytochemistry 2020, 178, 112460.| PDF
14. Zhao, W. #, Cong, Y. #, Li, H., Li, S., Shen, Y., Qi, Q., Zhang, Y., Li, Y., Tang, Y. * Challenges and potential for improving the druggability of podophyllotoxin-derived drugs in cancer chemotherapy. Nat. Prod. Rep. 2020, doi. 10.1039/d0np00041h.| PDF
2019
1. Xu, H., Liang, W., Ning, L., Jiang, Y., Yang, W., Wang, C., Qi, F., Ma, L., Du, L., Fourage, L., Zhou, Y. J. and Li, S.* Directed Evolution of P450 Fatty Acid Decarboxylases via High-Throughput Screening towards Improved Catalytic Activity. ChemCatChem 2019, 11, 1-6. | PDF
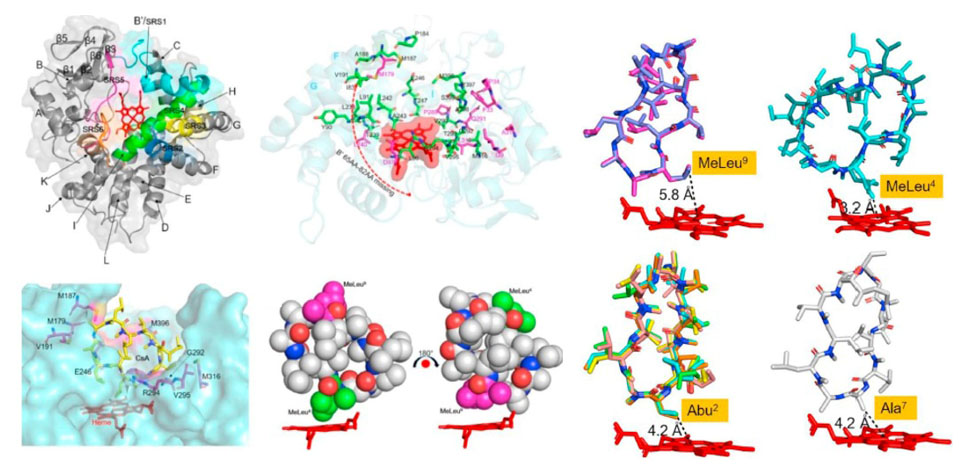
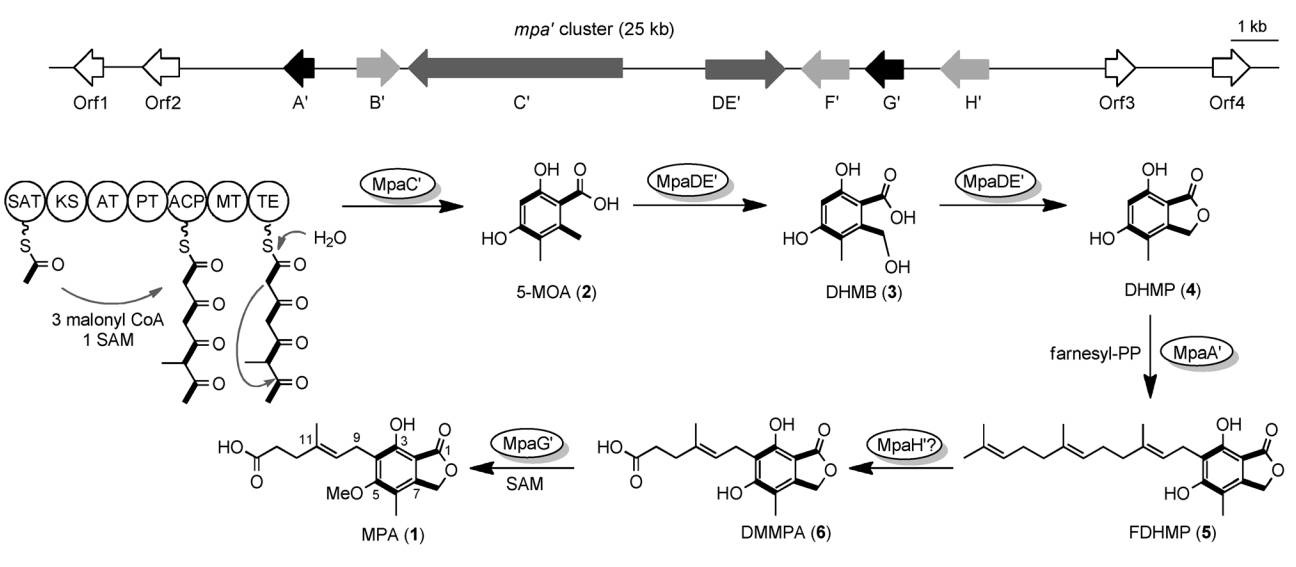
 2. Zhang, W., Du, L., Qu, Z., Zhang, X., Li, F., Li, Z., Qi, F., Wang, X., Jiang, Y., Men, P., Sun, J., Cao, S., Geng, C., Qi, F., Wan, X., Liu, C., and Li, S.* Compartmentalized Biosynthesis of Mycophenolic Acid. Proc. Natl. Acad. Sci. U.S.A. 2019, 116(27), 13305-13310. | PDF
2. Zhang, W., Du, L., Qu, Z., Zhang, X., Li, F., Li, Z., Qi, F., Wang, X., Jiang, Y., Men, P., Sun, J., Cao, S., Geng, C., Qi, F., Wan, X., Liu, C., and Li, S.* Compartmentalized Biosynthesis of Mycophenolic Acid. Proc. Natl. Acad. Sci. U.S.A. 2019, 116(27), 13305-13310. | PDF
3. Jiang, Y., Li, Z., Wang, C., Zhou, Y., Xu, H.*, and Li, S.* Biochemical characterization of three new α-olefn-producing P450 fatty acid decarboxylases with a halophilic property. Biotechnol. Biofuels 2019, 12, 79. | PDF
4. Huang, Y., Zheng, X., Pilgaard, B., Holck, J., Muschiol, J., Li, S. and Lange, L. Identification and characterization of GH11 xylanase and GH43 xylosidase from the chytridiomycetous fungus, Rhizophlyctis rosea. Appl. Microbiol. Biotechnol. 2019, 103, 777-791. | PDF
5. Jia, K., Zhu, L., Qu, X., Li, S., Shen, Y., Qi, Q., Zhang, Y., Li, Y., and Tang, Y.* Enzymatic o‑glycosylation of etoposide aglycone by exploration of the substrate promiscuity for glycosyltransferases. ACS Synth. Biol. 2019, 8(12), 2718-2725. | PDF
6. Duan, X.#, Ma, X.#, Li, S., and Zhou, Y.* Free fatty acids promote transformation eficiency of yeast. FEMS Yeast Res. 2019, 19(7), foz069. | PDF

3. Jiang, Y., Li, Z., Wang, C., Zhou, Y., Xu, H.*, and Li, S.* Biochemical characterization of three new α-olefn-producing P450 fatty acid decarboxylases with a halophilic property. Biotechnol. Biofuels 2019, 12, 79. | PDF
4. Huang, Y., Zheng, X., Pilgaard, B., Holck, J., Muschiol, J., Li, S. and Lange, L. Identification and characterization of GH11 xylanase and GH43 xylosidase from the chytridiomycetous fungus, Rhizophlyctis rosea. Appl. Microbiol. Biotechnol. 2019, 103, 777-791. | PDF
5. Jia, K., Zhu, L., Qu, X., Li, S., Shen, Y., Qi, Q., Zhang, Y., Li, Y., and Tang, Y.* Enzymatic o‑glycosylation of etoposide aglycone by exploration of the substrate promiscuity for glycosyltransferases. ACS Synth. Biol. 2019, 8(12), 2718-2725. | PDF
6. Duan, X.#, Ma, X.#, Li, S., and Zhou, Y.* Free fatty acids promote transformation eficiency of yeast. FEMS Yeast Res. 2019, 19(7), foz069. | PDF
2018
1. Zhang, W. #, Du, L. #, Li, F., Zhang, X., Qu, Z., Han, L., Li, Z., Sun, J., Qi, F., Yao, Q., Sun, Y., Geng, C., Li, S.* Mechanistic Insights into Interactions between Bacterial Class I P450 Enzymes and Redox Partners. ACS Catal. 2018, 8, 9992-10003. | PDF
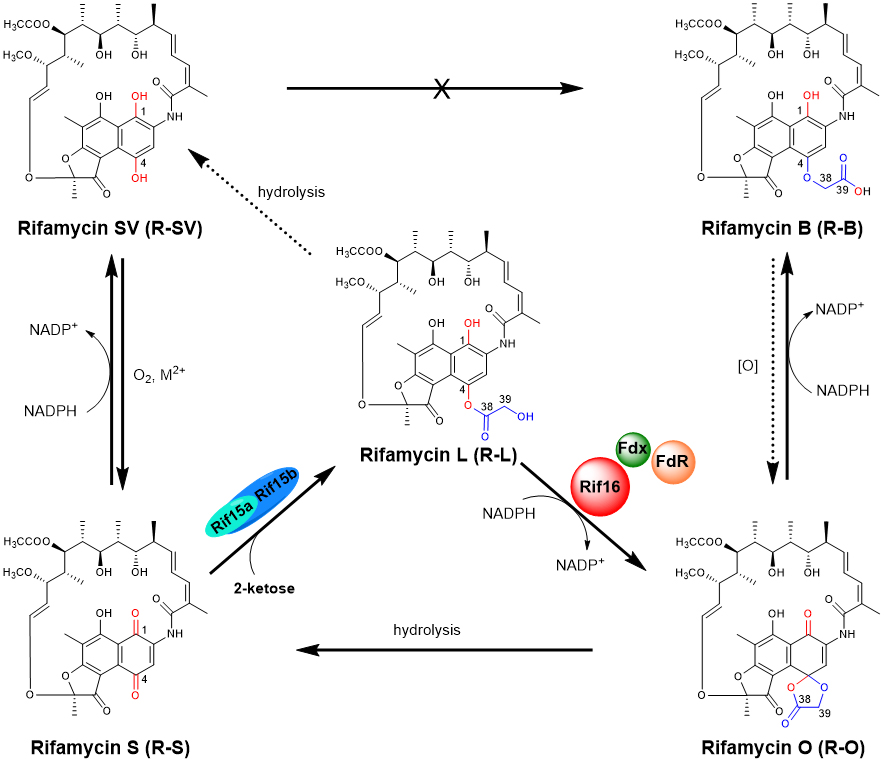
2. Qi, F. #, Lei, C. #, Li, F., Zhang, X., Wang, J., Zhang, W., Fan, Z., Li, W., Tang, G., Xiao, Y.*, Zhao, G., Li, S. * Deciphering the late steps of rifamycin biosynthesis. Nat. Commun. 2018, 9, 2342. | PDF
3. Chen, H., Meng, X., Xu, X., Liu, W., Li, S. * The molecular basis for lipase stereoselectivity. Appl. Microbiol. Biotechnol. 2018, 102(8), 3487-3495. | PDF
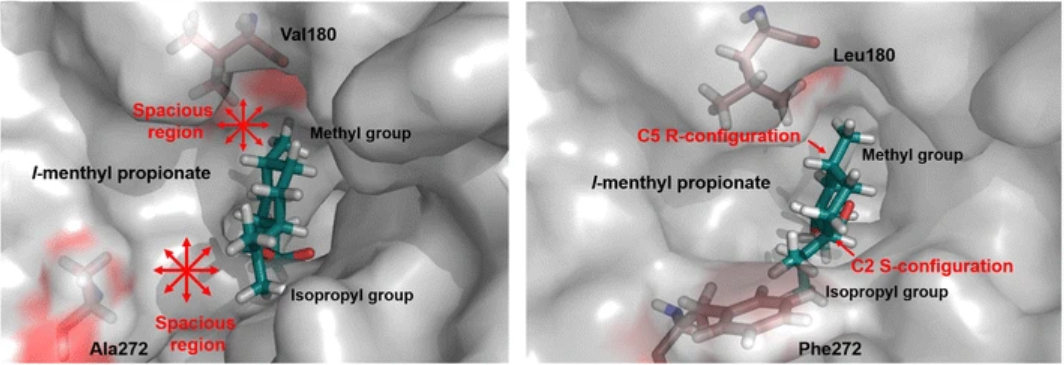
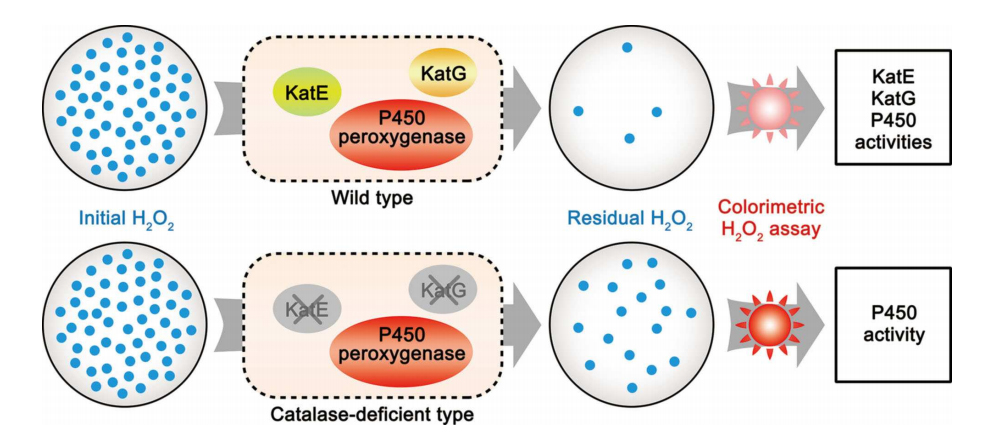
4. Jiang, Y., Li, S.* The catalytic function and application of cytochrome P450 enzymes in biosynthesis and organic synthesis. Chin. J. Org. Chem. 2018, 38, 2307-2323. | PDF
5. Zhang, X. #, Li, P. #, Qin, G., Li, S., Voogd, J. N., Tang, X.*, and Li, G.* Isolation and Absolute Configurations of Diversiform C17, C21 and C25 Terpenoids from the Marine Sponge Cacospongia sp. Mar. Drugs 2018, 17(1), 14. | PDF
6. Zhong,.B., Du, L., Li, Z., Zhang, W., Zhang, X., Li, J., Huang, S., Hu, X.*, Li, S., Liu, K.* Biosynthesis of Gastrodin Based on the Cytochrome P450-Mediated Oxidative Biodegradation of 4-Cresol. J. Hunan Normal Univ. (Natural Science) 2018, 41(4), 73-80. | PDF
7. Li, Q.*, Ding, W., Yao, Z., Tu, J., Wang, L., Huang, H., Li, S., and Ju, J.* AbmV Catalyzes Tandem Ether Installation and Hydroxylation during Neoabyssomicin/Abyssomicin Biosynthesis. Org. Lett. 2018, 20, 4854-4857. | PDF
8. Huang, Y., Zheng, X., Pilgaard, B., Holck, J., Muschiol, J., Li, S., and Lange, L.* Identification and characterization of GH11 xylanase and GH43 xylosidase from the chytridiomycetous fungus, Rhizophlyctis rosea. Appl. Microbiol. Biotechnol. 2018, 103(2), 777-791. | PDF
9. Dai, Z., Wang, Y., Zhou, Z., Li, S., Zhang, X *. Synthetic Biology for Production of Plant-derived Natural Products. Bull. Chin. Acad. Sci. 2018, 33(11), 1228-1238. | PDF
2. Qi, F. #, Lei, C. #, Li, F., Zhang, X., Wang, J., Zhang, W., Fan, Z., Li, W., Tang, G., Xiao, Y.*, Zhao, G., Li, S. * Deciphering the late steps of rifamycin biosynthesis. Nat. Commun. 2018, 9, 2342. | PDF
3. Chen, H., Meng, X., Xu, X., Liu, W., Li, S. * The molecular basis for lipase stereoselectivity. Appl. Microbiol. Biotechnol. 2018, 102(8), 3487-3495. | PDF

4. Jiang, Y., Li, S.* The catalytic function and application of cytochrome P450 enzymes in biosynthesis and organic synthesis. Chin. J. Org. Chem. 2018, 38, 2307-2323. | PDF
5. Zhang, X. #, Li, P. #, Qin, G., Li, S., Voogd, J. N., Tang, X.*, and Li, G.* Isolation and Absolute Configurations of Diversiform C17, C21 and C25 Terpenoids from the Marine Sponge Cacospongia sp. Mar. Drugs 2018, 17(1), 14. | PDF
6. Zhong,.B., Du, L., Li, Z., Zhang, W., Zhang, X., Li, J., Huang, S., Hu, X.*, Li, S., Liu, K.* Biosynthesis of Gastrodin Based on the Cytochrome P450-Mediated Oxidative Biodegradation of 4-Cresol. J. Hunan Normal Univ. (Natural Science) 2018, 41(4), 73-80. | PDF
7. Li, Q.*, Ding, W., Yao, Z., Tu, J., Wang, L., Huang, H., Li, S., and Ju, J.* AbmV Catalyzes Tandem Ether Installation and Hydroxylation during Neoabyssomicin/Abyssomicin Biosynthesis. Org. Lett. 2018, 20, 4854-4857. | PDF
8. Huang, Y., Zheng, X., Pilgaard, B., Holck, J., Muschiol, J., Li, S., and Lange, L.* Identification and characterization of GH11 xylanase and GH43 xylosidase from the chytridiomycetous fungus, Rhizophlyctis rosea. Appl. Microbiol. Biotechnol. 2018, 103(2), 777-791. | PDF
9. Dai, Z., Wang, Y., Zhou, Z., Li, S., Zhang, X *. Synthetic Biology for Production of Plant-derived Natural Products. Bull. Chin. Acad. Sci. 2018, 33(11), 1228-1238. | PDF
2017
1. Zhang, X., and Li, S.* Expansion of chemical space for natural products by uncommon P450 reactions. Nat. Prod. Rep. 2017, 34(9), 1061-1089. | PDF
2. Du, L. #, Dong, S. #, Zhang, X., Jiang, C., Chen, J., Yao, L., Wang, X., Wan, X., Liu, X., Wang, X., Huang, S., Cui, Q., Feng, Y.*, Liu, S.*, and Li, S.* Selective oxidation of aliphatic C–H bonds in alkylphenols by a chemomimetic biocatalytic system. Proc. Natl. Acad. Sci. U.S.A. 2017, 114(26), E5129-E5137. | PDF
3. Li, Z., Du, L., Zhang, W., Zhang, X., Jiang, Y., Liu, K., Men, P., Xu, H., Fortman, J. L., Sherman, D. H., Yu, B., Gao, S., Li, S.* Complete elucidation of the late steps of bafilomycin biosynthesis in Streptomyces lohii. J. Biol. Chem. 2017, 292(17), 7095-7104. | PDF
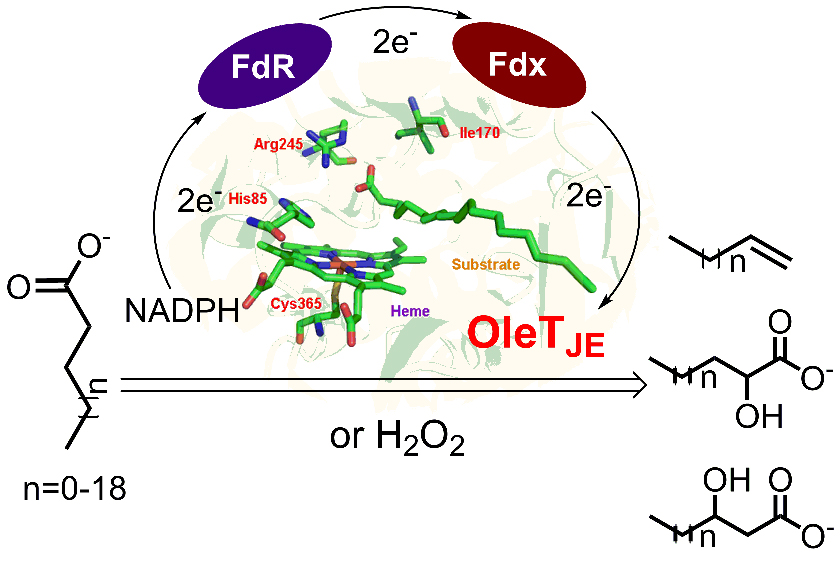
4. Fang, B. #, Xu, H. #, Liu, Y., Qi, F., Zhang, W., Chen, H., Wang, C., Wang, Y., Yang, W., and Li, S.* Mutagenesis and redox partners analysis of the P450 fatty acid decarboxylase OleTJE. Sci. Rep. 2017, 7, 44258. | PDF
5. Xu, H., Ning, L., Yang, W., Fang, B., Wang, C., Wang, Y., Xu, J., Colin, S., Laeuffer, F., Fourage, L., and Li, S. * In vitro oxidative decarboxylation of free fatty acids to terminal alkenes by two new P450 peroxygenases. Biotechnol. Biofuels 2017, 10, 208. | PDF
6. Sun, Y., Ma, L., Chen, H., Xu, H., Zheng, X., Qi, F., Li, S.* Production of α-alkenes catalyzed by the fused lipase and P450 fatty acid de-carboxylase. Chin. Sci. Bull. 2017, 62(1), 1-7. | PDF
7. Yao, Q. #, Ma, Li. #, Liu, L., Ikeda, H., Fushinobu, S., Li, S., and Xu, L.* Hydroxylation of Compactin (ML-236B) by CYP105D7 (SAV_7469) from Streptomyces avermitilis. J. Microbiol. Biotechnol. 2017, 27(5), 956–964. | PDF
8. Zhang, W. #, Huffman, J. #, Li, S., Shen, Y.*, and Du, L.,* Unusual acylation of chloramphenicol in Lysobacter enzymogenes, a biocontrol agent with intrinsic resistance to multiple antibiotics. BMC Biol. 2017, 17, 59-66. | PDF
9. Zhang, W., Zhao, B., Du, L.*, Shen, Y.* Cytotoxic Polyketides with an Oxygen-Bridged Cyclooctadiene Core Skeleton from the Mangrove Endophytic Fungus Phomosis sp. A818. Molecules. 2017, 22, 1547. | PDF
2. Du, L. #, Dong, S. #, Zhang, X., Jiang, C., Chen, J., Yao, L., Wang, X., Wan, X., Liu, X., Wang, X., Huang, S., Cui, Q., Feng, Y.*, Liu, S.*, and Li, S.* Selective oxidation of aliphatic C–H bonds in alkylphenols by a chemomimetic biocatalytic system. Proc. Natl. Acad. Sci. U.S.A. 2017, 114(26), E5129-E5137. | PDF
3. Li, Z., Du, L., Zhang, W., Zhang, X., Jiang, Y., Liu, K., Men, P., Xu, H., Fortman, J. L., Sherman, D. H., Yu, B., Gao, S., Li, S.* Complete elucidation of the late steps of bafilomycin biosynthesis in Streptomyces lohii. J. Biol. Chem. 2017, 292(17), 7095-7104. | PDF

4. Fang, B. #, Xu, H. #, Liu, Y., Qi, F., Zhang, W., Chen, H., Wang, C., Wang, Y., Yang, W., and Li, S.* Mutagenesis and redox partners analysis of the P450 fatty acid decarboxylase OleTJE. Sci. Rep. 2017, 7, 44258. | PDF

5. Xu, H., Ning, L., Yang, W., Fang, B., Wang, C., Wang, Y., Xu, J., Colin, S., Laeuffer, F., Fourage, L., and Li, S. * In vitro oxidative decarboxylation of free fatty acids to terminal alkenes by two new P450 peroxygenases. Biotechnol. Biofuels 2017, 10, 208. | PDF
6. Sun, Y., Ma, L., Chen, H., Xu, H., Zheng, X., Qi, F., Li, S.* Production of α-alkenes catalyzed by the fused lipase and P450 fatty acid de-carboxylase. Chin. Sci. Bull. 2017, 62(1), 1-7. | PDF
7. Yao, Q. #, Ma, Li. #, Liu, L., Ikeda, H., Fushinobu, S., Li, S., and Xu, L.* Hydroxylation of Compactin (ML-236B) by CYP105D7 (SAV_7469) from Streptomyces avermitilis. J. Microbiol. Biotechnol. 2017, 27(5), 956–964. | PDF
8. Zhang, W. #, Huffman, J. #, Li, S., Shen, Y.*, and Du, L.,* Unusual acylation of chloramphenicol in Lysobacter enzymogenes, a biocontrol agent with intrinsic resistance to multiple antibiotics. BMC Biol. 2017, 17, 59-66. | PDF
9. Zhang, W., Zhao, B., Du, L.*, Shen, Y.* Cytotoxic Polyketides with an Oxygen-Bridged Cyclooctadiene Core Skeleton from the Mangrove Endophytic Fungus Phomosis sp. A818. Molecules. 2017, 22, 1547. | PDF
2016
1. Sun, Y. #, Ma, L. #, Han, D. #, Du, L., Qi, F., Zhang, W., Sun, J., Huang, S., Kim, E.-S.,* and Li, S.* In vitro reconstitution of the cyclosporine specific P450 hydroxylases using heterologous redox partner proteins. J. Ind. Microbiol. Biotechnol. 2016, 44(2), 161-166. | PDF
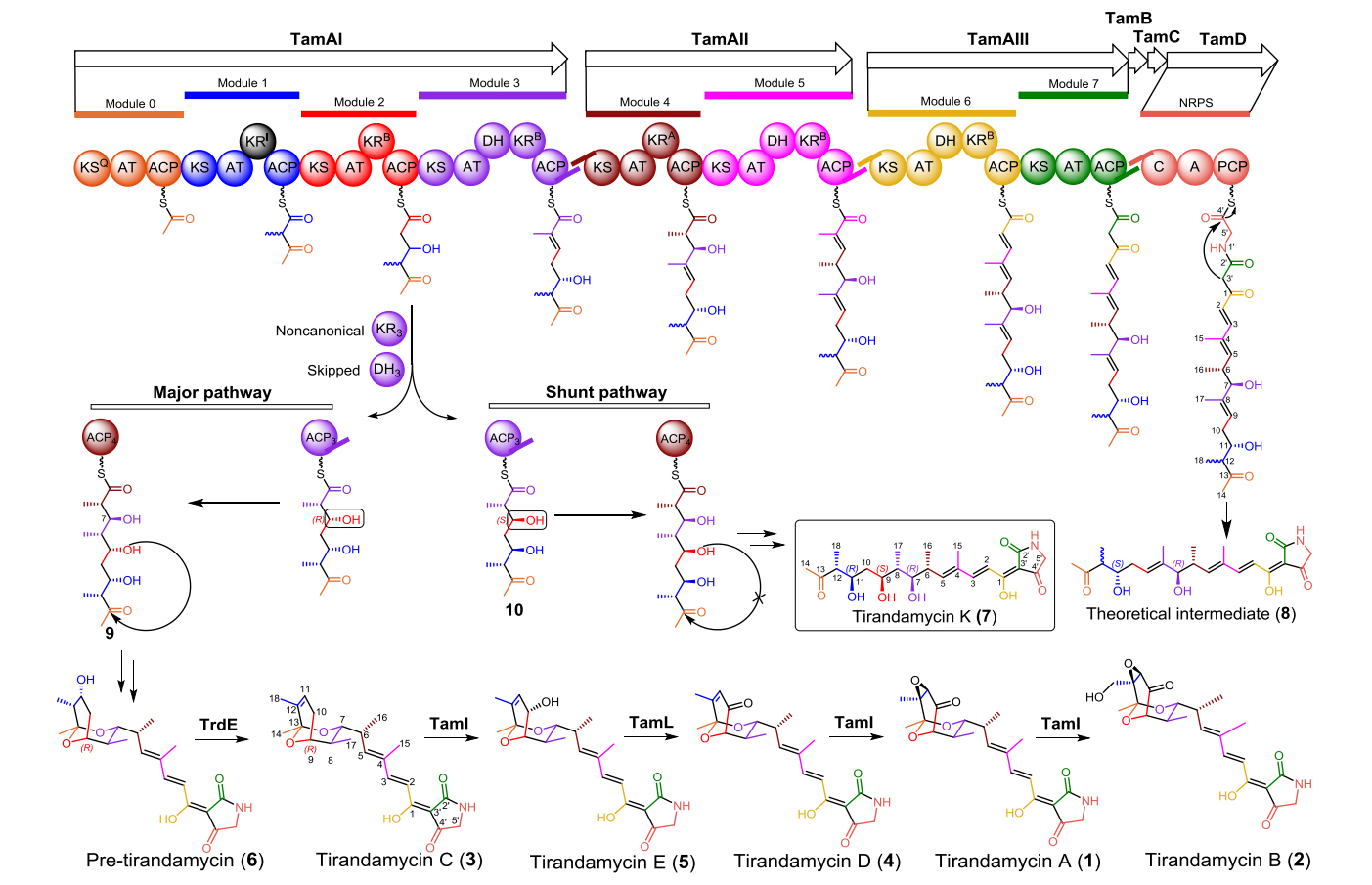
2. Zhang, X., Li, Z., Du, L., Chlipala, G. E., Lopez, P. C., Zhang, W., Sherman, D. H.*, and Li, S.* Identification of an unexpected shunt pathway product provides new insights into tirandamycin biosynthesis. Tetrahedron Lett. 2016, 57(52), 5919-5923. | PDF
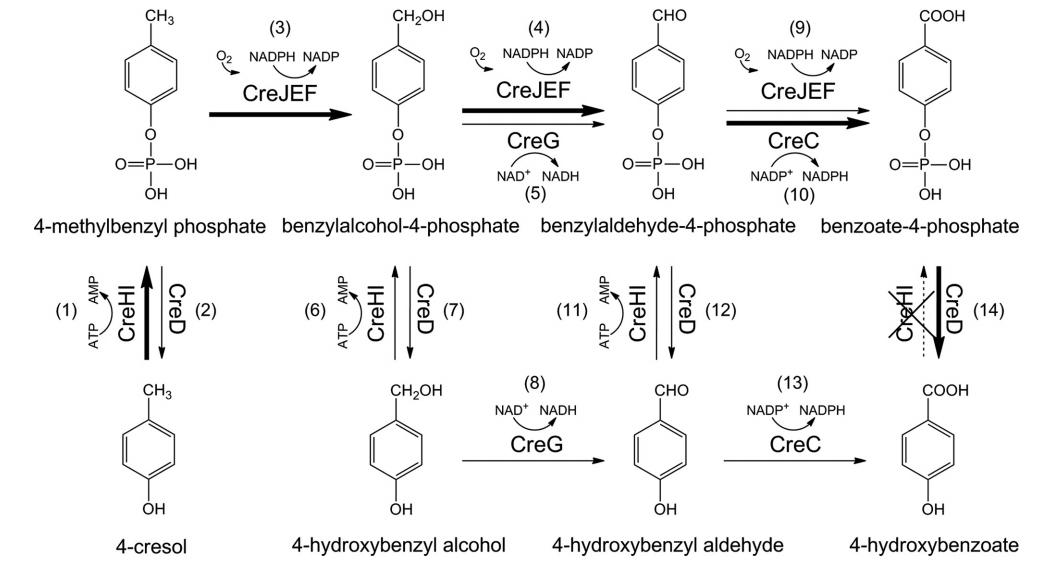
3. Du, L., Ma, L., Qi, F., Zheng, X., Jiang, C., Li, A., Wan, X., Liu, S.-J.*, and Li, S.* Characterization of a Unique Pathway for 4-Cresol Catabolism Initiated by Phosphorylation in Corynebacterium glutamicum. J. Biol. Chem. 2016, 291(12), 6583-6594. | PDF
4. Zheng, X., Fang, B., Han, D., Yang, W., Qi, F., Chen, H., and Li, S.* Improving the Secretory Expression of an α-Galactosidase from Aspergillus niger in Pichia pastoris. PLoS ONE 2016, 11(8), e0161529. | PDF
5. Li, Z., Zhang, W., and Li, S.* Cytochrome P450 enzymes and microbial drug development – A review. Acta Microbiol. Sin. 2016, 56(3), 496-515. | PDF
6. Sun, J., Liu, C., Li, R., Zhang, W. *, Li, S., Functional characterization of PbPT, a prenyltransferase from Penicillium Brevicompactum NRRL 864. J. Qingdao Univ. (E&T), 2016, 31(2),107-115. | PDF

2. Zhang, X., Li, Z., Du, L., Chlipala, G. E., Lopez, P. C., Zhang, W., Sherman, D. H.*, and Li, S.* Identification of an unexpected shunt pathway product provides new insights into tirandamycin biosynthesis. Tetrahedron Lett. 2016, 57(52), 5919-5923. | PDF

3. Du, L., Ma, L., Qi, F., Zheng, X., Jiang, C., Li, A., Wan, X., Liu, S.-J.*, and Li, S.* Characterization of a Unique Pathway for 4-Cresol Catabolism Initiated by Phosphorylation in Corynebacterium glutamicum. J. Biol. Chem. 2016, 291(12), 6583-6594. | PDF

4. Zheng, X., Fang, B., Han, D., Yang, W., Qi, F., Chen, H., and Li, S.* Improving the Secretory Expression of an α-Galactosidase from Aspergillus niger in Pichia pastoris. PLoS ONE 2016, 11(8), e0161529. | PDF
5. Li, Z., Zhang, W., and Li, S.* Cytochrome P450 enzymes and microbial drug development – A review. Acta Microbiol. Sin. 2016, 56(3), 496-515. | PDF
6. Sun, J., Liu, C., Li, R., Zhang, W. *, Li, S., Functional characterization of PbPT, a prenyltransferase from Penicillium Brevicompactum NRRL 864. J. Qingdao Univ. (E&T), 2016, 31(2),107-115. | PDF
2015
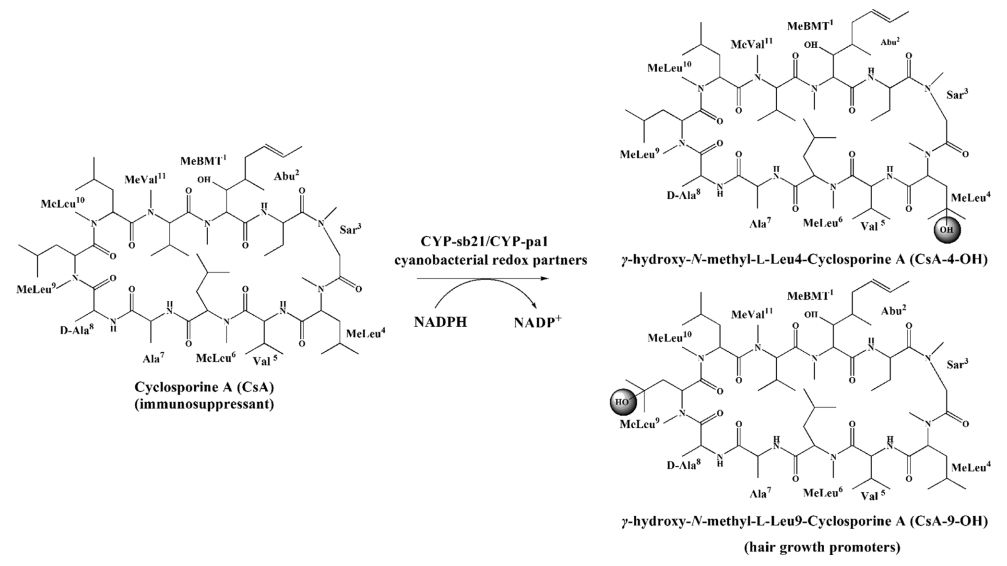
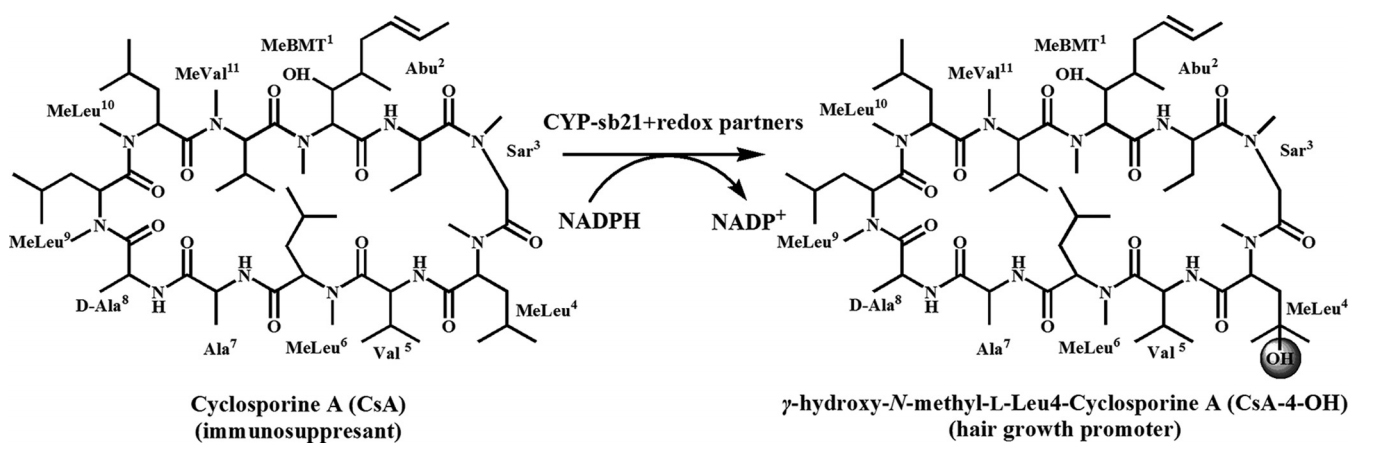
1. Ma, L., Du, L., Chen, H., Sun, Y., Huang, S., Zheng, X., Kim, E.-S.*, and Li, S.* Reconstitution of the in vitro activity of the cyclosporine-specific P450 hydroxylase from Sebekia benihana and development of a heterologous whole cell biotransformation system. Appl. Environ. Microbiol. 2015, 81(18), 6268-6275. | PDF
2. Yan, J.*, Liu, Y., Wang, C., Han, B., and Li, S.*Assembly of lipase and P450 fatty acid decarboxylase to constitute a novel biosynthetic pathway for production of 1-alkenes from renewable triacylglycerols and oils. Biotechnol. Biofuels 2015, 8, 34. | PDF

3. Zhang, W., Cao, S., Qiu, L., Qi, F., Li, Z., Yang, Y., Huang, S., Bai, F., Liu, C., Wan, X.,* and Li, S.* Functional Characterization of MpaG’, the O-Methyltransferase Involved in the Biosynthesis of Mycophenolic Acid. Chembiochem 2015, 16(4), 565-569. | PDF
4. Woo, M. W., Lee, B. R., Nah, H.J., Choi, S. S., Li, S., and Kim, E.-S.* Domain Characterization of Cyclosporin Regio-specific Hydroxylases in Rare Actinomycetes. J. Microbiol. Biotechnol. 2015, 25(10), 1634-1639. | PDF
5. Qi, G. #, Wang, D. #, Yu, L., Tang, X., Chai, G., He, G., Ma, W., Li, S., Kong, Y., Fu, C.*, and Zhou, G.* Metabolic engineering of 2-phenylethanol pathway producing fragrance chemical and reducing lignin in Arabidopsis. Plant Cell Rep. 2015, 34(8), 1331-1342. | PDF
6. Bernard, S. M., Akey, D. L., Tripathi, A., Park, S. R., Konwerski, J. R., Anzai, Y., Li, S., Kato, F., Sherman, D. H., and Smith, J. L.* Structural Basis of Substrate Specificity and Regiochemistry in the MycF/TylF Family of Sugar O-Methyltransferases. ACS Chem. Biol. 2015, 10(5), 1340-1351. | PDF

2. Yan, J.*, Liu, Y., Wang, C., Han, B., and Li, S.*Assembly of lipase and P450 fatty acid decarboxylase to constitute a novel biosynthetic pathway for production of 1-alkenes from renewable triacylglycerols and oils. Biotechnol. Biofuels 2015, 8, 34. | PDF

3. Zhang, W., Cao, S., Qiu, L., Qi, F., Li, Z., Yang, Y., Huang, S., Bai, F., Liu, C., Wan, X.,* and Li, S.* Functional Characterization of MpaG’, the O-Methyltransferase Involved in the Biosynthesis of Mycophenolic Acid. Chembiochem 2015, 16(4), 565-569. | PDF

4. Woo, M. W., Lee, B. R., Nah, H.J., Choi, S. S., Li, S., and Kim, E.-S.* Domain Characterization of Cyclosporin Regio-specific Hydroxylases in Rare Actinomycetes. J. Microbiol. Biotechnol. 2015, 25(10), 1634-1639. | PDF
5. Qi, G. #, Wang, D. #, Yu, L., Tang, X., Chai, G., He, G., Ma, W., Li, S., Kong, Y., Fu, C.*, and Zhou, G.* Metabolic engineering of 2-phenylethanol pathway producing fragrance chemical and reducing lignin in Arabidopsis. Plant Cell Rep. 2015, 34(8), 1331-1342. | PDF
6. Bernard, S. M., Akey, D. L., Tripathi, A., Park, S. R., Konwerski, J. R., Anzai, Y., Li, S., Kato, F., Sherman, D. H., and Smith, J. L.* Structural Basis of Substrate Specificity and Regiochemistry in the MycF/TylF Family of Sugar O-Methyltransferases. ACS Chem. Biol. 2015, 10(5), 1340-1351. | PDF
2014
1. Yan, J.*, Zheng, X., Du, L., and Li, S.* Integrated lipase production and in situ biodiesel synthesis in a recombinant Pichia pastoris yeast: an efficient dual biocatalytic system composed of cell free enzymes and whole cell catalysts. Biotechnol. Biofuels 2014, 7, 55. | PDF

2. Zhang, W., Liu, Y., Yan, J., Cao, S., Bai, F., Yang, Y., Huang, S., Yao, L., Anzai, Y., Kato, F., Podust, L. M., Sherman, D. H.*, and Li, S.* New reactions and products resulting from alternative interactions between the P450 enzyme and redox partners. J. Am. Chem. Soc. 2014, 136(9), 3640-3646. (Faculty of 1000 Prime: http://f1000.com/prime/718276150) | PDF
3. Liu, Y., Wang, C., Yan, J., Zhang, W., Guan, W., Lu, X., and Li, S.* Hydrogen peroxide-independent production of α-alkenes by OleTJE P450 fatty acid decarboxylase. Biotechnol. Biofuels 2014, 7, 28. | PDF
4. Yan, J.*, Zheng, X., and Li, S.* A novel and robust recombinant Pichia pastoris yeast whole cell biocatalyst with intracellular overexpression of a Thermomyces lanuginosus lipase: preparation, characterization and application in biodiesel production. Bioresour. Technol. 2014, 151, 43-48. | PDF
5. Zhang, W. #, Xu, L. #, Yang, L., Huang, Y., Li, S., and Shen. Y.* Phomopsidone A, a novel depsidone metabolite from the mangrove endophytic fungus Phomopsis sp. A123. Fitoterapia 2014, 96, 146-151. | PDF
6. Huang, S.*, Gao, J., Wu, R., Li, S. and Bai Z.* Polydimethylsiloxane: A General Matrix for High-Performance Chromatographic NMR Spectroscopy. Angew. Chem. Intl. Ed. 2014, 53, 11592-11595. | PDF
7. Wang, Q. #, Huang, X. #, Zhang, J., Lu, X., Li, S., and Li, J.-J.* Engineering self-sufficient aldehyde deformylating oxygenase fused to alternative electron transfer systems for efficient conversion of aldehydes into alkanes. Chem. Comm. 2014, 50, 4299-4301. | PDF

2. Zhang, W., Liu, Y., Yan, J., Cao, S., Bai, F., Yang, Y., Huang, S., Yao, L., Anzai, Y., Kato, F., Podust, L. M., Sherman, D. H.*, and Li, S.* New reactions and products resulting from alternative interactions between the P450 enzyme and redox partners. J. Am. Chem. Soc. 2014, 136(9), 3640-3646. (Faculty of 1000 Prime: http://f1000.com/prime/718276150) | PDF
3. Liu, Y., Wang, C., Yan, J., Zhang, W., Guan, W., Lu, X., and Li, S.* Hydrogen peroxide-independent production of α-alkenes by OleTJE P450 fatty acid decarboxylase. Biotechnol. Biofuels 2014, 7, 28. | PDF
4. Yan, J.*, Zheng, X., and Li, S.* A novel and robust recombinant Pichia pastoris yeast whole cell biocatalyst with intracellular overexpression of a Thermomyces lanuginosus lipase: preparation, characterization and application in biodiesel production. Bioresour. Technol. 2014, 151, 43-48. | PDF
5. Zhang, W. #, Xu, L. #, Yang, L., Huang, Y., Li, S., and Shen. Y.* Phomopsidone A, a novel depsidone metabolite from the mangrove endophytic fungus Phomopsis sp. A123. Fitoterapia 2014, 96, 146-151. | PDF
6. Huang, S.*, Gao, J., Wu, R., Li, S. and Bai Z.* Polydimethylsiloxane: A General Matrix for High-Performance Chromatographic NMR Spectroscopy. Angew. Chem. Intl. Ed. 2014, 53, 11592-11595. | PDF
7. Wang, Q. #, Huang, X. #, Zhang, J., Lu, X., Li, S., and Li, J.-J.* Engineering self-sufficient aldehyde deformylating oxygenase fused to alternative electron transfer systems for efficient conversion of aldehydes into alkanes. Chem. Comm. 2014, 50, 4299-4301. | PDF
2013
1. Zhang W. #, Fortman, J. L.#, Carlson, J. C., Yan, J., Liu, Y., Bai, F., Guan, W., Jia, J., Matainaho, T., Sherman, D. H.*, and Li, S.* Characterization of the bafilomycin biosynthetic gene cluster from Streptomyces lohii. Chembiochem, 2013, 14(3), 301-306. | PDF
2. Sunderhaus, J. D., McAfoos, T. J., Finefield, J. M., Kato, H., Li, S., Tsukamoto, S., Sherman, D. H., and Williams, R. M.* Synthesis and bioconversions of notoamide T: a biosynthetic precursor to stephacidin A and notoamide B. Org. Lett. 2013, 15(1), 22-25. | PDF
2. Sunderhaus, J. D., McAfoos, T. J., Finefield, J. M., Kato, H., Li, S., Tsukamoto, S., Sherman, D. H., and Williams, R. M.* Synthesis and bioconversions of notoamide T: a biosynthetic precursor to stephacidin A and notoamide B. Org. Lett. 2013, 15(1), 22-25. | PDF
2012
1. Li, S., Tietz, D. R., Rutaganira, F. U., Kells, P. M., Anzai, Y., Kato, F., Pochapsky, T. C., Sherman, D. H., and Podust, L. M.* Substrate recognition by the multifunctional cytochrome P450 MycG in mycinamicin hydroxylation and epoxidation reactions. J. Biol. Chem. 2012, 287(45), 37880-37890. | PDF
2. Li, S., Anand, K., Tran, H., Yu, F., Finefield, J. M., Sunderhaus, J. D., McAfoos, T. J., Tsukamoto, S., Williams, R. M.*, and Sherman, D. H.* Comparative analysis of the biosynthetic systems for fungal bicyclo[2.2.2]diazaoctane indole alkaloids: the (+)/(-)-notoamide, paraherquamide and malbrancheamide pathways. MedChemComm 2012, 3(8), 987-996. | PDF
3. Li, S., Finefield, J. M., Sunderhaus, J. D., McAfoos, T. J., Williams, R. M.*, and Sherman, D. H.* Biochemical characterization of NotB as an FAD-dependent oxidase in the biosynthesis of notoamide indole alkaloids. J. Am. Chem. Soc. 2012, 134(2), 788-791. | PDF
4. Anzai, Y.*, Tsukada, S. I., Sakai, A., Masuda, R., Harada, C., Domeki, A., Li, S., Kinoshita, K., Sherman, D. H., and Kato, F. Function of the cytochrome P450 enzymes MycCI and MycG in Micromonospora griseorubida, a producer of the macrolide antibiotic mycinamicin. Antimicrob. Agents Chemother. 2012, 56(7), 3648-3656. | PDF
2. Li, S., Anand, K., Tran, H., Yu, F., Finefield, J. M., Sunderhaus, J. D., McAfoos, T. J., Tsukamoto, S., Williams, R. M.*, and Sherman, D. H.* Comparative analysis of the biosynthetic systems for fungal bicyclo[2.2.2]diazaoctane indole alkaloids: the (+)/(-)-notoamide, paraherquamide and malbrancheamide pathways. MedChemComm 2012, 3(8), 987-996. | PDF
3. Li, S., Finefield, J. M., Sunderhaus, J. D., McAfoos, T. J., Williams, R. M.*, and Sherman, D. H.* Biochemical characterization of NotB as an FAD-dependent oxidase in the biosynthesis of notoamide indole alkaloids. J. Am. Chem. Soc. 2012, 134(2), 788-791. | PDF
4. Anzai, Y.*, Tsukada, S. I., Sakai, A., Masuda, R., Harada, C., Domeki, A., Li, S., Kinoshita, K., Sherman, D. H., and Kato, F. Function of the cytochrome P450 enzymes MycCI and MycG in Micromonospora griseorubida, a producer of the macrolide antibiotic mycinamicin. Antimicrob. Agents Chemother. 2012, 56(7), 3648-3656. | PDF
2011
1. Carlson, J. C.#, Li, S. #, Gunatilleke, S. S., Anzai, Y., Burr, D. A., Podust, L. M., and Sherman, D. H.* Tirandamycin biosynthesis is mediated by co-dependent oxidative enzymes. Nat. Chem. 2011, 3(8), 628-633. | PDF
2. Akey, D. L., Li, S., Konwerski, J., Confer, L., Bernard, S. M., Anzai, Y., Kato, F., Sherman, D. H., and Smith, J. A.* New structural form in the SAM/metal-dependent O-methyltransferase family: MycE from the mycinamycin biosynthetic pathway. J. Mol. Biol. 2011, 413(2), 438-450. | PDF
2. Akey, D. L., Li, S., Konwerski, J., Confer, L., Bernard, S. M., Anzai, Y., Kato, F., Sherman, D. H., and Smith, J. A.* New structural form in the SAM/metal-dependent O-methyltransferase family: MycE from the mycinamycin biosynthetic pathway. J. Mol. Biol. 2011, 413(2), 438-450. | PDF
2010
1. Ding, Y., de Wet, J. R., Cavalcoli, J., Li, S., Greshock, T. J., Miller, K. A., Finefield, J. M., Sunderhaus, J. D., McAfoos, T. J., Tsukamoto, S., Williams, R. M.*, and Sherman, D. H.* Genome-based characterization of two prenylation steps in the assembly of the stephacidin and notoamide anticancer agents in a marine-derived Aspergillus sp. J. Am. Chem. Soc. 2010, 132(36), 12733-12740. | PDF
2. McAfoos, T. J., Li, S., Tsukamoto, S., Sherman, D. H., and Williams, R. M.* Studies on the Biosynthesis of the stephacidins and notoamides. Total synthesis of notoamides. Heterocycles 2010, 82(1), 461-472. | PDF
3. Tsukada, S., Anzai, Y., Li, S., Kinoshita, K., Sherman, D. H., and Kato, F.* Gene targeting for O-methyltransferase genes, mycE and mycF, on the chromosome of Micromonospora griseorubida producing mycinamicin with a disruption cassette containing the bacteriophage φC31 attB attachment site. FEMS Microbiol. Lett. 2010, 304(2), 148-156. | PDF
4. Carlson, J. C., Fortman, J. L., Anzai, Y., Li, S., Douglas, A. B., and Sherman, D. H.* Identification of the tirandamycin biosynthetic gene cluster from Streptomyces sp. 307-9. Chembiochem 2010, 11(4), 564-572. | PDF
2. McAfoos, T. J., Li, S., Tsukamoto, S., Sherman, D. H., and Williams, R. M.* Studies on the Biosynthesis of the stephacidins and notoamides. Total synthesis of notoamides. Heterocycles 2010, 82(1), 461-472. | PDF
3. Tsukada, S., Anzai, Y., Li, S., Kinoshita, K., Sherman, D. H., and Kato, F.* Gene targeting for O-methyltransferase genes, mycE and mycF, on the chromosome of Micromonospora griseorubida producing mycinamicin with a disruption cassette containing the bacteriophage φC31 attB attachment site. FEMS Microbiol. Lett. 2010, 304(2), 148-156. | PDF
4. Carlson, J. C., Fortman, J. L., Anzai, Y., Li, S., Douglas, A. B., and Sherman, D. H.* Identification of the tirandamycin biosynthetic gene cluster from Streptomyces sp. 307-9. Chembiochem 2010, 11(4), 564-572. | PDF
2009
1. Li, S., Chaulagain, M. R., Knauff, A. R., Podust, L. M., Montgomery, J.*, and Sherman, D. H.* Selective oxidation of carbolide C-H bonds by an engineered macrolide P450 monooxygenase. Proc. Natl. Acad. Sci. U.S.A. 2009, 106(44), 18463-18468. | PDF
2. Carlson, J. C. #, Li, S.#, Douglas, A. B., and Sherman, D. H.* Isolation and characterization of tirandamycins from a marine-derived Streptomyces sp. J. Nat. Prod. 2009, 72(11), 2076-2079. (*Co-first authors) | PDF
3. Li, S., Anzai, Y., Kinoshita, K., Kato, F., Sherman, D. H.* Functional analysis of MycE and MycF, two O-methyltransferases involved in the biosynthesis of mycinamicin macrolide antibiotics. Chembiochem 2009, 10(8), 1297-1301. | PDF
4. Li, S., Ouellet, H., Sherman, D. H., Podust, L. M.* Analysis of transient and catalytic desosamine-binding pockets in cytochrome P-450 PikC from Streptomyces venezuelae. J. Biol. Chem. 2009, 284(9), 5723-5730. | PDF
2. Carlson, J. C. #, Li, S.#, Douglas, A. B., and Sherman, D. H.* Isolation and characterization of tirandamycins from a marine-derived Streptomyces sp. J. Nat. Prod. 2009, 72(11), 2076-2079. (*Co-first authors) | PDF
3. Li, S., Anzai, Y., Kinoshita, K., Kato, F., Sherman, D. H.* Functional analysis of MycE and MycF, two O-methyltransferases involved in the biosynthesis of mycinamicin macrolide antibiotics. Chembiochem 2009, 10(8), 1297-1301. | PDF
4. Li, S., Ouellet, H., Sherman, D. H., Podust, L. M.* Analysis of transient and catalytic desosamine-binding pockets in cytochrome P-450 PikC from Streptomyces venezuelae. J. Biol. Chem. 2009, 284(9), 5723-5730. | PDF
2008
1. Anzai, Y. #, Li, S. #, Chaulagain, M. R., Kinoshita, K., Kato, F., Montgomery, J., Sherman, D. H.* Functional analysis of MycCI and MycG, cytochrome P450 enzymes involved in biosynthesis of mycinamicin macrolide antibiotics. Chem. Biol. 2008, 15(9), 950-959. | PDF
2007
1. Li, S., Podust, L. M., Sherman, D. H.* Engineering and analysis of a self-sufficient biosynthetic cytochrome P450 PikC fused to the RhFRED reductase domain. J. Am. Chem. Soc. 2007, 129(43), 12940-12941. | PDF
2. Li, S., Grüschow, S., Dordick, J. S., Sherman, D. H.* Molecular analysis of the role of tyrosine 224 in the active site of Streptomyces coelicolor RppA, a bacterial type III polyketide synthase. J. Biol. Chem. 2007, 282(17), 12765-12772. | PDF
2. Li, S., Grüschow, S., Dordick, J. S., Sherman, D. H.* Molecular analysis of the role of tyrosine 224 in the active site of Streptomyces coelicolor RppA, a bacterial type III polyketide synthase. J. Biol. Chem. 2007, 282(17), 12765-12772. | PDF
2006
1. Sherman, D. H.*, Li, S., Yermalitskaya, L. V., Kim, Y., Smith, J. A., Waterman, M. R., Podust, L. M.* The structural basis for substrate anchoring, active site selectivity, and product formation by P450 PikC from Streptomyces venezuelae. J. Biol. Chem. 2006, 281(36), 26289-26297. | PDF
2004
1. Zhang, J., Zheng, Z., Huang, Y., Li, S., Su, W.* Preliminary research on antitumor activities of natural products from algal fungi. J. Xiamen Univ. (Natural Science) 2004, 43 (4), 551-556. | PDF
2003
1. Li, S., Wang, J.*, Zheng, Z., Xu, Q., Huang, Y., Zhao, Y., Su, W. 1-(2,4-Dihydroxy-3,5-Dimethylphenyl)-ethanone (Clavatol). Acta. Cryst. 2003, E59, o1649-o1650.
2. Li, S., Zheng, Z.* Discovery and development of marine antitumor natural products. Mar. Sci. Bull. 2003, 22(2), 76-82. | PDF
2. Li, S., Zheng, Z.* Discovery and development of marine antitumor natural products. Mar. Sci. Bull. 2003, 22(2), 76-82. | PDF
PATENTS
1. Li Shengying, David H. Sherman. Chimeric Cytochrome P450 Proteins and Methods of Use. (US 20090081758A3)
2. Li Shengying, David H. Sherman, Mani Raj Chaulagain, John Montgomery, and Allison R. Knauff. A Method for Selective Oxidation of C-H Bonds by an Engineered P450 Enzyme. (WO 2011038313A2)
3. Li Shengying, David H. Sherman, Krithika Anand, and Robert M. Williams. Biosynthetic Systems Producing Fungal Indole Alkaloids. (US 9650656 B2)
4. Yan, Jinyong, Li Shengying. The invention relates to a method for catalyzing the production of biodiesel by coupling lipase production.(201410138507.3)
5. Li Shengying, Liu Yi, Lv Xuefeng, and Liu Xufeng. The invention relates to a method for producing terminal olefins and its application.(201410660346.4)
6. Yan Jinyong, Li Shengying. The invention relates to aliphatic olefin catalytic synthesis method based on coupling catalysis of lipase and P450 fatty acid decarboxylase.(ZL201510003451.5)
7. Li Shengying, Ma Li, and Kim Eung-Soo. The invention relates to a biological transformation method for producing hair growth promoter.(201510346350.8)
8. Li Shengying, Du lei, and Liu Shuangjiang. The invention relates to a catalytic oxidation system of phenolic compounds and its application.(201610015698.3)
9. Li Shengying, Zheng Xianliang. The construction and application of engineering bacteria with high secretion and expression of Aspergillus Niger alpha-galactosidase AGA.(2015107066150)
10. Li Shengying, Fang Bo, Qi Fengxia, Zhang Wei, and Du Lei. The invention relates to combination of redox partners to support the activity of P450 fatty acid decarboxylase and its application.(201610151612.X)
11. Fourage Laurent, Laeuffer Frederie, Strub Henri, Wang Yun, Xu Jian, Xu Huifang, and Li Shengying. Production of Alpha-Olefins. (WO 2017001606A1)
12. Li Shengying, Xu Huifang, and Ning Linlin. Method for detecting hydrogen peroxide conversion activity of an enzyme. ( EP17290115.9)
13. Li Shengying, Xu Huifang, and Ning Linlin. Improved cytochrome P450 fatty acid decarboxylases. ( EP18290089.4)
14. Li Shengying, Li Zhong, Du Lei, Zhang Xingwang, and Zhang Wei. The invention relates to the construction of an engineering bacteria with high yield of pavloamycin and its application.(201811634377.7)
2. Li Shengying, David H. Sherman, Mani Raj Chaulagain, John Montgomery, and Allison R. Knauff. A Method for Selective Oxidation of C-H Bonds by an Engineered P450 Enzyme. (WO 2011038313A2)
3. Li Shengying, David H. Sherman, Krithika Anand, and Robert M. Williams. Biosynthetic Systems Producing Fungal Indole Alkaloids. (US 9650656 B2)
4. Yan, Jinyong, Li Shengying. The invention relates to a method for catalyzing the production of biodiesel by coupling lipase production.(201410138507.3)
5. Li Shengying, Liu Yi, Lv Xuefeng, and Liu Xufeng. The invention relates to a method for producing terminal olefins and its application.(201410660346.4)
6. Yan Jinyong, Li Shengying. The invention relates to aliphatic olefin catalytic synthesis method based on coupling catalysis of lipase and P450 fatty acid decarboxylase.(ZL201510003451.5)
7. Li Shengying, Ma Li, and Kim Eung-Soo. The invention relates to a biological transformation method for producing hair growth promoter.(201510346350.8)
8. Li Shengying, Du lei, and Liu Shuangjiang. The invention relates to a catalytic oxidation system of phenolic compounds and its application.(201610015698.3)
9. Li Shengying, Zheng Xianliang. The construction and application of engineering bacteria with high secretion and expression of Aspergillus Niger alpha-galactosidase AGA.(2015107066150)
10. Li Shengying, Fang Bo, Qi Fengxia, Zhang Wei, and Du Lei. The invention relates to combination of redox partners to support the activity of P450 fatty acid decarboxylase and its application.(201610151612.X)
11. Fourage Laurent, Laeuffer Frederie, Strub Henri, Wang Yun, Xu Jian, Xu Huifang, and Li Shengying. Production of Alpha-Olefins. (WO 2017001606A1)
12. Li Shengying, Xu Huifang, and Ning Linlin. Method for detecting hydrogen peroxide conversion activity of an enzyme. ( EP17290115.9)
13. Li Shengying, Xu Huifang, and Ning Linlin. Improved cytochrome P450 fatty acid decarboxylases. ( EP18290089.4)
14. Li Shengying, Li Zhong, Du Lei, Zhang Xingwang, and Zhang Wei. The invention relates to the construction of an engineering bacteria with high yield of pavloamycin and its application.(201811634377.7)